Research advances on the multiple uses of
Moringa oleifera: A sustainable alternative for ocially
neglected population
Raimunda Samia Nogueira Brilhante1, Jamille Alencar Sales , Vandbergue Santos Pereira
Débora de Souza Collares Maia Castelo-Branco , Rossana de Aguiar Cordeiro , Célia Maria de Souza SampaioManoel de Araújo Neto Paiva , João Bosco Feitosa dos Santos , José Júlio Costa Sidrim , Marcos F abio Gadelha Rocha
Department of Pathology and Legal Medicine, Postgraduate Program in Medical Microbiology, Specialized Medical Mycology Center, Federal University of Cear ´a, Fortaleza, Ceara, Brazil
School of Veterinary Medicine, Postgraduate Program in Veterinary Sciences, State University of Ceara, Fortaleza, Ceara, Brazil
Department of Social Sciences, Postgraduate Program of Sociology, State University of Ceara, Fortaleza, Ceara, Brazil ´
ARTICLE INFO
ABSTRACT
Article history:
Received 28 Dec 2016
Received in revised form 15 Apr 2017
Accepted 25 May 2017
Available online 28 Jul 2017
Keywords:
Moringa oleifera
Biotechnology
Nutrition
Health
Aquaculture
Moringa oleifera Lam (Moringaceae) is a plant with high nutritional and medicinal value. Native to India, it is now widely distributed throughout tropical and subtropical regions of the world. Its different parts are sources of proteins, vitamins and minerals and present different pharmacological and biotechnological potential. Moreover, M. oleifera seeds are widely used in water and effluent treatment, for their coagulation, flocculation and sedimentation properties, their ability of improving ater quality, by reducing organic matter and microbial load, with special applicability in intensive animal production systems, such as aquaculture. In addition, due to its high nutritional value and several medicinal properties, this tree may act as a nutritional and medical alternative for socially neglected populations. In this context, this review gathers information on M. oleifera, emphasizing its chemical constituents, nutritional, pharmacological and antimicrobial properties, applications in the treatment of water effluents, and ecological and social aspects.
1. Introduction
Medicinal plants have posed as natural resources of compounds with pharmacological and nutritional properties aiding humans to prevent and treat diseases . Among several plants evaluated in bioprospective studies, Moringa oleifera (Lam) (M. oleifera), popularly known, in Brazil, as “moringa”, “lírio branco” or “quiabo-de-quina”, and, in some parts of the world, as drumstick tree or horseradish tree, has stood out in alternative medical therapies, showing benefits for the control of several diseases . Its medicinal potential derives from
First and corresponding author: Raimunda Samia Nogueira Brilhante, Rua Coronel Nunes de Melo. 1315, Rodolfo Teo´filo, 60420-270, Fortaleza, CE, Brazil.
Tel: +55 (85) 3366 8319.
E-mail: brilhante@ufc.br
Peer review under responsibility of Hainan Medical University.
Foundation project: This work was supported by grants from the National Council for Scientific and Technological Development (CNPq; Brazil; Processes
307606/2013-9; 443167/2014-1) and Coordination Office for the Improvement of Higher Education Personnel (AEI-0052-000650100/11).
secondary metabolites, such as alkaloids, tannins, flavonoids, steroids, saponins, coumarins, quinones and resins . M. oleifera is native to Northern India, but currently it is widely distributed in the Americas, Africa, Europe, Oceania and Asia . Leaves, flowers, pods and seeds of this tree are considered a food source of high nutritional value in the African continent and other countries, particularly in India, Philippines and Pakistan Three nongovernmental organizations, Trees for Life, Church World Service, and the Educational Concerns for Hunger Organization, have advocated the motto “Natural nutrition for the tropics” to stimulate the use of several plant species as food sources, including M. oleifera . Leaves can be consumed cooked or fresh and they can be stored as dried powder unrefrigerated with no nutritional losses, for several months. Undoubtedly, M. oleifera adds substantial health benefits to countries where hunger is a problem . In addition to medicinal and nutritional applications, one of the most applied properties of M. oleifera is the highly efficient coagulating effect of its seeds, which are used in wate
1995-7645/Copyright © 2017 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
treatment. These seeds act as coagulants of organic matter suspended in water and are used in water treatment stations for natural cleaning before erforming other cleansing processes. Furthermore, seeds have more stable activity in different pH ranges, when compared to aluminum sulfate, the most frequently used coagulating substance in water treatment stations . M. oleifera has also been assessed for its potential to treat aquaculture waste water. The results have shown the simultaneous elimination of water turbidity, suspended particles and microorganisms, making it suitable to completely or partly replace the usual coagulating agents, leading to economic, health and environmental gains
Based on the above, this review gathers information on M. oleifera (Lam.), emphasizing its chemical constituents, nutritional, pharmacological and antimicrobial properties, applications in the treatment of water effluents, and ecological and social aspects
2. Chemical constituents
The chemical constituents of M. oleifera stems, leaves, flowers, pods and seeds have been analyzed for the presence of bioactive compounds, demonstrating the predominance of secondary metabolites, such as phenolic acids, gallic acid, ellagic acid, chlorogenic acid, ferulic acid, glucosinolates, flavonoids, quercetin, vanillin and kaempferol, which have nutritional, pharmaceutical and/or antimicrobial properties . However, the amount of these metabolites in M. oleifera extracts varies according to geographic location, soil, sun exposure and climatic conditions. Moreover, the method and solvents used for extraction can modify the content of the compounds obtained from the plant, mainly phenols and flavonoids Many phytoconstituents of M. oleifera have been isolated and studied, as shown in Table 1. The main phytochemicals obtained from the plant include: tannins, saponins, alkaloids, flavonoids, phenols and glycosides from leaves tannins, steroids, flavonoids, alkaloids, glycosides, quercetin and terpenoids from flowers gallic acid, catechins, epicatechin, ferulic acid, vanillin, caffeic acid, protocatecuic acid, cinnamic
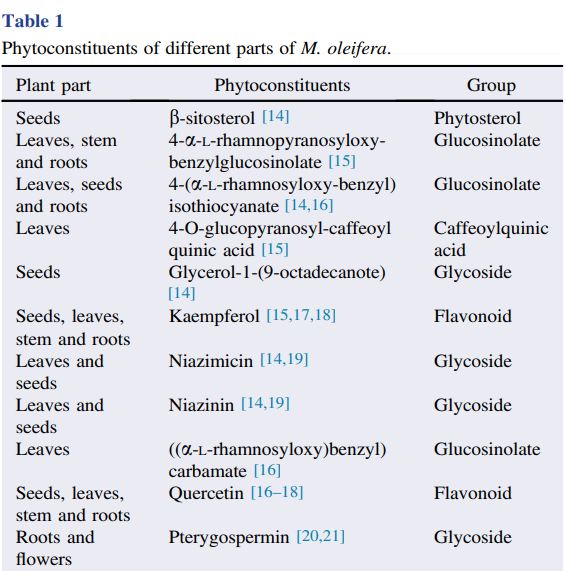
acid, phytosterol, quercetin, glycosides and phenols from seeds procyanidins, aurantiamide acetate, 3-dibenzylurea, quercetin glycoside, rhamnoglucoside quercetin, and hlorogenic acid from roots; and procyanidin, sterols, triterpenoids, glycosides, tannins, alkaloids, b-sitosterol and octacosanoic acid from stem bark .
3. M. oleifera and its applications
3.1. Nutritional potential
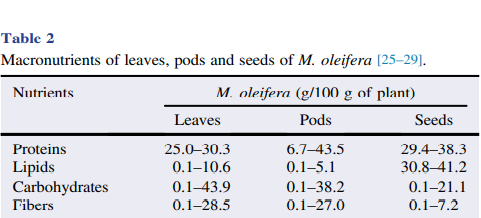
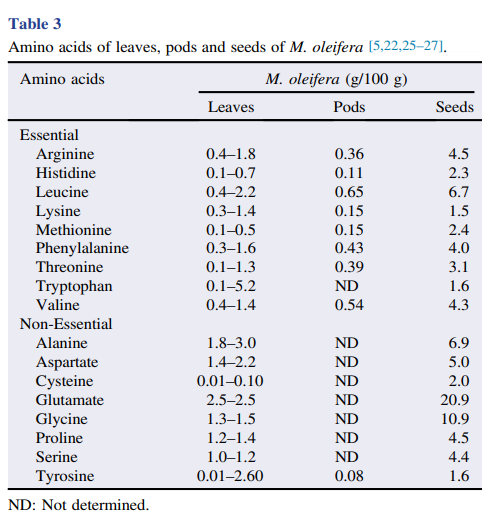
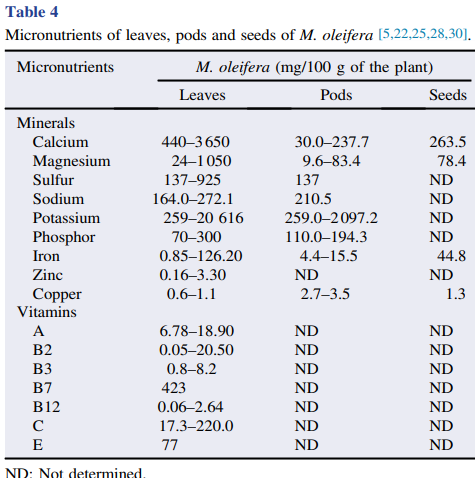
M. oleifera contains more than 90 nutritional chemical compounds, including proteins, lipids, carbohydrates and dietary fibers (Table 2). It is used in the tropics as a food source to overcome malnutrition, especially in children and infants . Among the several nutrients found in different parts of M. oleifera, proteins are the most abundant, accounting for approximately 25% of dry weight, and at least 19 amino acids have been identified in this plant (Table 3). Furthermore, M. oleifera also contains several minerals and vitamins (Table 4). Lipids are abundant in seeds, mainly stearic acid, saturated palmitic acid and oleic acid, representing about 30% of dry weight. The lipidic compounds linolenic acid and palmitic acid are the main constituents of M. oleifera leaves. In addition, the high nutritional content found in dried leaves is an indicator of the usefulness of the plant as a food resource .
3.2. Hepato and nephro-protective activity
Scientific evidences suggest a potential role of M. oleifera leaves in the reduction of liver and kidney drug-induced damage in animals (Table 5). For instance, studies have reported the hepato and renal-protective properties of M. oleifera against several drugs, such as gentamicin, pyrazinamide, rifampicin, isoziazide and acetaminophen, which are mainly attributable to its leaves . The authors also observed a reduction in serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase , urea and creatinine in animals treated with M. oleifera leaf extract. These findings were confirmed by histological tests, which showed reduction of drug-induced hepatic and renal damage in animals treated with M. oleifera leaves. Additionally, aqueous and alcoholic root and flower extracts of M. oleifera have been shown to have hepatoprotective activity against the effects of acetaminophen, reducing serum transaminases (alanine aminotransferase and aspartate aminotransferase), alkaline phosphatase and bilirubin levels . In addition, this activity also enhances the recovery of cadmium-induced hepatotoxicity in rats . However, further studies are still needed to better define the pharmaceutical applicability of M. oleifera.
3.3. Hypocholesterolemic, hypolipidemic and antiatherosclerotic activity
Ghasi et al. observed a hypocholesterolemic activity after the administration of a crude extract of M. oleifera leaves to rats fed on a high-fat diet, causing a reduction of up to 14% in serum cholesterol levels . M. oleifera fruit consumption is also effective in reducing very-low-density lipoprotein, low-density lipoprotein and high-density lipoprotein serum levels . In addition to these effects, M. oleifera leaf extract has also been reported to reduce the formation of atherosclerotic plaques . Although there are only a few studies in humans, some researches have demonstrated the potential benefits of using M. oleifera for the treatment of hyperglycemia and dyslipidemia (Table 5). For instance, a study with 46 individuals with type-2 diabetes, daily treated with 8 g of M. oleifera leaf powder for 40 d, showed that fasting and postprandial glycemia were reduced by 28% and 26%, respectively, when compared to untreated individuals . In ddition, total cholesterol, triglycerides and low-density lipoprotein and very-low-density lipoprotein cholesterol were also lower than those of the control individuals . Another study with 35 type-2 diabetic individuals showed that the consumption of 4.6 g-tablets of M. oleifera leaves, for 50 d, was able to increase high-density lipoprotein levels and decrease total cholesterol .
3.4. Anticancer potential
In general, there are a few in vitro studies to evaluate the anticancer potential of M. oleifera (Table 5). However, the existing results suggest the potential anticancer properties of M. oleifera. One of the first studies on M. oleifera antitumor effect was performed with compounds obtained from its ethanol seed extract, showing that the compounds 4-(a-L-rhamnosyloxy)-benzyl isothiocyanate, 3-O-(60 -O-oleoyl-b-D-glucopyranosyl)-b-sitosterol, b-sitosterol-3-O-b-D-glucopyranoside and niazimicin are potent tumor inhibitors .
Dichloromethane and methanolic M. oleifera leaf extracts present in vitro anticancer activity against human hepatocellular carcinoma, colorectal adenocarcinoma and breast adenocarcinoma, with no toxic effects on human fibroblasts Other investigators studied the effects of oral administration of hydromethanolic and methanolic M. oleifera leaf extracts on a mouse melanoma model. The authors observed that the oral administration of 500 mg/kg, for 15 d, delayed tumor growth and significantly increased mouse lifespan . These anticancer properties may be attributed to the bioactive compounds present in these extracts, such as the hexadecanoic acidethyl ester .
3.5. Anti-inflammatory and immunomodulatory
activities
The anti-inflammatory activity of M. oleifera has been
observed after treatment with extracts of roots, stems, leaves, flowers, pods and seeds (Table 5). In a study with rats, M. oleifera root extract reduced the development of paw edema, with results similar to those obtained by phenylbutazone, a nonsteroidal anti-inflammatory drug with analgesic and antipyretic properties Furthermore, the butanol extract of M. oleifera seeds interrupted the acetylcholine-induced bronchospasms and airway inflammation in guinea pigs, by modifying Th1/Th2 cytokines . In addition, a clinical study with patients with mild to moderate asthma demonstrated that M. oleifera dried seed powder significantly improved the forced vital capacity, forced expiratory volume and peak expiratory flow without adverse reactions. Many bioactive compounds may be involved in the anti-inflammatory properties of M. oleifera, such as quercetin, which appears to inhibit the activation of NFkB, essential step to unchain the inflammatory process. However, many other bioactive compounds from M. oleifera, such as flavonoids and phenolic acids, may be involved in the anti-inflammatory activity of this plant. It has also been shown that M. oleifera leaf extract and quercetin regulate the expression of iNOS, IFN-g and C-reactive protein and decrease TNF-a and IL-6 release, in rats. A similar result was found for isothiocyanates obtained from M. oleifera leaves, which significantly decreased the production of pro-inflammatory mediators by RAW macrophages, especially IL-1b, iNOS, TNF-a and NO. Regarding the immunomodulatory effects of M. oleifera, it has been shown that the ethanolic M. oleifera leaf extract reduced cyclophosphamide-induced immunosuppression in rats, with stimulation of cellular and humoral immunity .
3.6. Antioxidant activity
The antioxidant activity of M. oleifera is particularly strong in leaf, pod and seed extracts (Table 5). The high content of flavonoids and phenols in different parts of the plant, especially leaves, favors the reduction of oxidative damage to the main biomolecules through the inhibition of lipid peroxidation and the action of nitric oxide and induction of deoxyribose degradation, preventing the generation of free radicals . Studies with normal and diabetic rats showed that treatment with aqueous M. oleifera leaf extracts significantly increased the activity of the enzymes superoxide dismutase, catalase and glutathione S-transferase and decreased lipid peroxidation It has been suggested that the high phenolic and flavonoid content in the extract may protect against oxidative damage in normal and diabetic individuals. Additionally, a research with 60 postmenopausal women showed that supplementation with M. oleifera leaf powder for 3 months significantly decreased the serum levels of malondialdehyde, generated by lipid peroxidation, and increased the levels of ascorbic acid, superoxide dismutase and glutathione peroxidase, which are indicators of the antioxidant property of the plant.
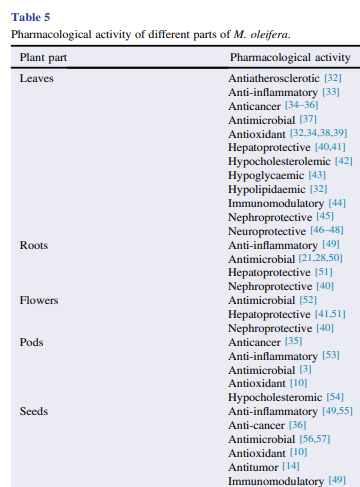
3.7. Neuroprotective potential
The neuroprotective effects of M. oleifera are an emerging area of study (Table 5). It has been shown that aqueous and hydroalcoholic extracts of M. oleifera leaves potentiate the cognitive activity, besides acting as neuroprotector in mice with colinotoxininduced dementia . Reduced levels of brain lipid peroxidation and increased levels of superoxide dismutase and catalase were observed in response to leaf extract administration . In addition, another study has demonstrated the neuroprotective properties of an ethanolic extract of M. oleifera leaves, when incubated with a primary culture of hippocampal neurons. The extract promoted neurite outgrowth with significant increase in the number and length of dendrites and axonal branches . These results suggest that M. oleifera may provide a neuroprotective benefit by reducing the oxidative stress.
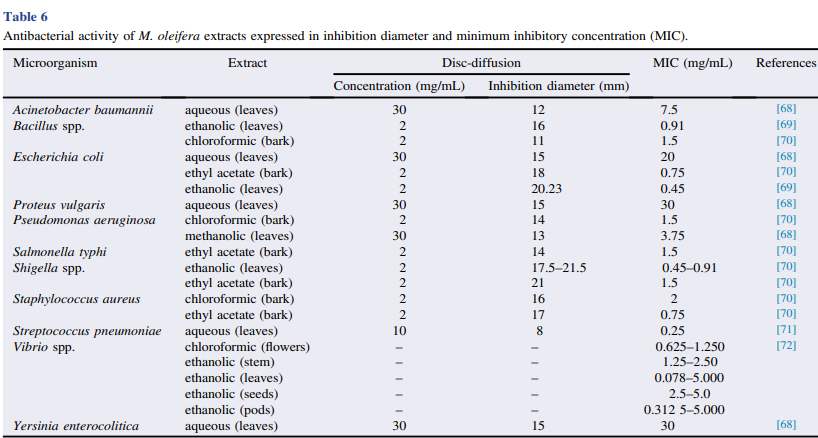
3.8. Antimicrobial potential
Many in vitro studies have demonstrated the inhibitory activity of M. oleifera root, stem, leaf, flower, pod and seed extracts on Gram-positive (Enterococcus faecalis, methicillinresistant Staphylococcus aureus and Staphylococcus epidermidis) and Gram-negative bacteria (Salmonella enterica, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli) isolated from clinical samples . The antimicrobial effect of the crude extracts on E. coli and K. pneumoniae strains has been compared to that of the antibiotic streptomycin . The in vitro antibacterial potential of M. oleifera has been demonstrated against other bacterial species, as shown in Table 6. This potential is associated with the biocompound benzyl-isothiocyanate which inhibits bacterial growth by disrupting the mechanisms of membrane and enzyme synthesis . In addition, the antibacterial activity of M. oleifera extracts is also attributed to gallic acid and tannins, which inhibit Vibrio spp. , and saponins, tannins, isothiocya nates and phenolic compounds, such as alkaloids and flavonoids, which have inhibitory activity .
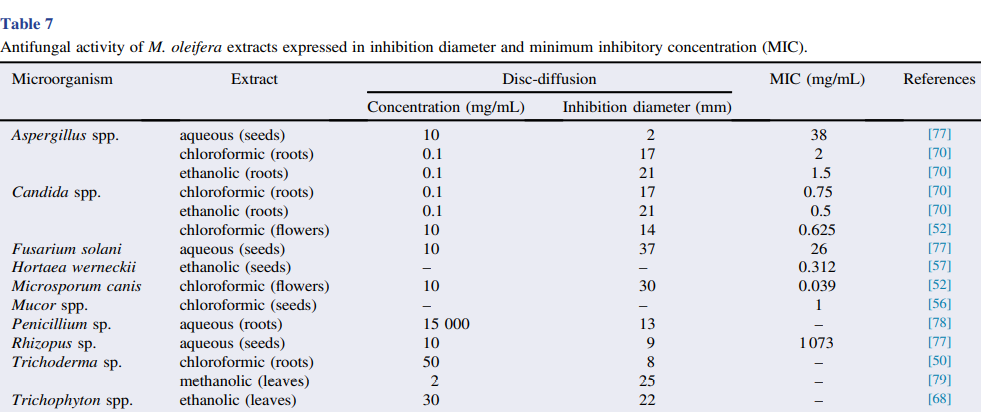
Several studies have demonstrated the antifungal activity of M. oleifera seed extracts against Mucor spp. and Rhizopus sp.; pod extracts against Alternaria sp., Colletotrichum sp., Candida albicans and Fusarium sp.; and root extracts against C. albicans and Aspergillus flavus . Other fungal species are also susceptible to M. oleifera seed and leaf extracts, such as the dermatophytes Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum and Microsporum canis isolated from clinical samples as well as Candida species, such as C. famata, C. guilliermondii, C. parapsilosis sensu lato, C. tropicalis and C. ciferri isolated from prawn farming . M. canis isolated from cases of feline dermatophytosis, as well as C. albicans from the oral microbiota of dogs were also susceptible to flower and seed extracts The in vitro antifungal effects of M. oleifera have also been demonstrated on other fungal species, as shown in Table 7. In addition to these extracts, seed essential oil has an inhibitory effect on Penicillium spp. and Aspergillus niger. The antifungal activity of this essential oil was attributed to polyphenols, hydrocarbons, hexacosane, pentacosane, heptacosane, phytol and thymol Moreover, flavonoids and the compounds pyterigospermin and isothiocyanates obtained from seeds and leaves also have antimicrobial activity .In addition to the inhibitory effects on planktonic bacteria and fungi, M. oleifera seeds also have antimicrobial activity against biofilms of microorganisms of clinical interest, such as S. aureus and P. aeruginosa and the yeast C. albicans. The biocompounds possibly involved in this activity are saponins, tannins, isothiocyanates and phenolic compounds, such as alkaloids, and, especially, flavonoids, which are present at high concentrations in seeds . The antiviral potential of ethanol extract of M. oleifera seeds was reported against human herpesvirus-4, called the Epstein– Barr virus and herpes simplex virus type 1 . In addition, hydroalcoholic leaf extracts inhibit hepatitis B virus replication , and silver nanoparticles synthesized using M. oleifera seed extract as reducing and stabilizing agent have inhibitory activity against dengue virus type 2 . The major biocompounds associated with antiviral activity are isocyanate and niaziminin. Despite these reports, there are still few studies on the antiviral potential of M. oleifera.
3.9. Insect control
M. oleifera seeds, leaves and flowers present insecticidal, larvicidal and ovicidal activity against the vectors of the species Anopheles stephensi and Aedes aegypti. The larvicidal activity of proteins, such as the water-soluble M. oleifera lectin obtained from seeds, has been demonstrated against organophosphate-resistant stage four A. aegypti larvae. However, the environmental use of M. oleifera products against insects is still questioned, due to toxicity to the green alga Scenedesmus obliquus and the crustacean Daphnia magna, which are commonly used to evaluate the toxicity of pollutants . In addition, according to Prabhu et al. , the bioefficiency of leaf and seed extracts of this plant can be demonstrated by spraying these extracts in breeding foci of A. stephensi, with larvicidal effect on different stages of development, as well as toxicity to the adult stage. Moreover, the aqueous extract of M. oleifera seeds is active against A. aegypti larvae and the methanol root extract is effective for controlling the mosquitoes Culex quinquefasciatus and Aedes albopictus, vectors of nematodes and viruses of public health importance, respectively .

4. Use of M. oleifera in water and effluent treatment
Frequently, the water used for human consumption is subjected to physical and chemical procedures to make it drinkable. In a treatment station, water passes through coagulation and flocculation processes which use chemical coagulants, such as aluminum sulfate and ferric chloride. However, M. oleifera seeds can be used as natural coagulants to treat water effluents in urban and rural areas for clarification, reduction of microbial load and control of helminths, such as Schistosoma mansoni. Moreover, seeds are also used to regulate the pH and control the microbial load in the treatment of water for human consumption . M. oleifera seeds have been found to promote 90% reduction in turbidity and color of contaminated water, and 90%–99% reduction in the bacterial load .
The coagulant activity of M. oleifera seeds is associated with their water-soluble lectin, which is responsible for their flocculating and sedimenting properties. Seeds reduce turbidity, microparticle content and microbial load, ergo, they are suitable coagulant agents that can replace other commonly used coagulants, such as aluminum sulfate and other organo-synthetic polymers which may be harmful to human, animal and environmental health. In a comparative study, M. oleifera seeds were cheaper and more effective than aluminum sulfate in reducing the turbidity of contaminated water, causing up to 95% decrease in turbidity, while aluminum sulfate caused an 80% reduction. In 2001, Okuda et al. demonstrated that aluminum sulfate is an efficient coagulant only within a certain pH range, while M. oleifera seeds act independently of pH, constituting an additional advantage in poorer regions where controlling the pH of drinking water before the coagulation process is seldom possible .
The analysis of the chemical constitution of M. oleifera seeds reveals that the pulp contains low molecular weight proteins and the process of dissolving the pulp in aqueous solutions constitutes an active network that favors colloid aggregation and the adsorption of metal ions [3,92,93]. In a recent study, the effectiveness of coagulation/flocculation using M. oleifera seeds was demonstrated by the reduction of turbidity and chemical concentration of biocompounds . In another research, the activity of the seeds in the biodegradation of benzene, toluene, ethylbenzene, p-xylene and o-xylene was identified, with the additional benefit of preserving medium pH and optimizing contact time, when compared to commercial activated charcoal, which is commonly used in cleaning systems of industrial effluent water .
Coagulant proteins from M. oleifera seeds are also able to reduce bacterial load. In this context, an isolated cationic protein is used for water treatment in some developing countries, hence, its use in antimicrobial therapeutic applications has been proposed. According to Shebek et al., this M. oleifera cationic protein fuses the inner and outer membranes of E. coli cells .
In addition to seeds, biomass obtained from barks has also been suggested as a promising low-cost compound for water effluent treatment, as it has been shown to adsorb heavy metals from farm solid waste .
5. Applications of M. oleifera in aquaculture
Aquaculture in many countries is under strong political and social pressure to reduce environmental damage caused by intensive production systems, as a result of the use of chemicals and antibiotics for water treatment and disease prevention and control.
The use of antimicrobial drugs poses a risk to human and animal health, as an intensive selective pressure for microbial communities, and favors environmental contamination by chemical residues . In this context, M. oleifera potentially represents an alternative for aquaculture, since this plant is a source of coagulant, antioxidant, and antimicrobial agents. Suspensions obtained from M. oleifera crushed seeds reduce organic matter and turbidity, due to the activity of the protein of the seed extract, which eliminates humic acids from water, improving water quality. In addition, as previously described, these seeds promote sedimentation or suspension and reduce bacterial load in contaminated water .
Some studies have reported the potential use of crude, ethanol and aqueous extracts of M. oleifera for water treatment and reduction of microbial load in fish and shrimp farming . Antimicrobial effects of M. oleifera seed extracts have been demonstrated against S. aureus, E. coli and V. cholerae isolated from tilapia (Oreochromis niloticus) and the shrimp Litopenaeus vannamei farming . Moreover, ethanol extract of leaves, pods and seeds, and chloroform extract of flowers have shown antimicrobial activity against microorganisms recovered from Macrobrachium amazonicum prawn farming, such as V. cholerae serogroups non-O1, non-O139, V. mimicus and V. vulnificus, as well as Candida spp. (C. ciferri, C. famata, C. guilliermondii, C. parapsilosis and C. tropicalis), and the dematiaceous filamentous fungus H. werneckii .
The antimicrobial potential of M. oleifera against bacteria and fungi recovered from aquatic animal farming deserves attention because these micro-organisms are potentially zoonotic opportunistic pathogens that may cause economic losses in aquaculture, as well as public health problems . Most of these pathogens are not necessarily associated with the farmed animals, once they are also commonly found in the water where the animals are kept. Thus, it is important to emphasize that water acts as an important vehicle for the spread of these microorganisms, demonstrating the importance of properly treating water effluents from aquaculture .
Considering the potential applications of M. oleifera products in aquaculture, the toxicity of M. oleifera extracts to cultivated shrimp was investigated , showing that flower, leaf and stem extracts are not toxic to M. amazonicum prawns at concentrations of up to 200 mg/mL . Therefore, the use of M. oleifera is advocated for aquaculture water treatment and microbial control, with reduced risks of harming human, animal and environmental health. Moreover, besides the inhibitory effects of M. oleifera on different microorganisms, it has also been reported that crude extracts of M. oleifera leaves and seeds also inhibit microbial protease activity, which is responsible for muscular degradation of fish and shrimp during storage . Thus, the potential use of these extracts as seafood preservatives to control their proteolysis and deterioration, during low temperature storage, has been suggested In this context, the perspective of using M. oleifera products in wastewater treatment and microbial control in aquaculture, as well as in seafood conservation, represents an environmental friendly approach to reduce the impacts of aquaculture on the environment and public health
6. Ecological aspects of M. oleifera
M. oleifera is considered ecologically viable for its several applications as an alternative to chemically developed products, reducing the risks associated with the accumulation of nonbiodegradable chemical compounds that are harmful to human, animal and environmental health. Among the potential applications of the plant, the coagulating, flocculating and adsorbing properties are remarkable for their ability to clean contaminated water, reducing its turbidity, toxicity and microbial load .
The treatment of water effluents with M. oleifera seeds has been proposed as a cheaper and more effective alternative to the use of aluminum sulfate, especially in rural areas, where the economical status and the accessibility to these products are key elements for maintaining the standards of fresh water treatment. Additionally, the use of M. oleifera seeds avoids the residual accumulation of chemical agents and maintains the optimum water pH values, after removing water turbidity, without requiring sophisticated equipments for pH dosing, nor special facilities for the treatment of drinking water . After treating effluent water with M. oleifera seeds, the sludge obtained after sedimentation, along with the seeds, can be used as biofertilizers, representing an additional benefit in rural areas.
Other studies have also shown the biosorbent properties of the biomass of seed husks, seeds and pods of M. oleifera in water contaminated with lead, a heavy metal that is toxic to humans and animals and harmful to the environment. Moreover, the use of M. oleifera seeds in the treatment of effluents from coffee fermentation has been proposed as an ecologically viable alternative, once coffee production generates residual water rich in organic nutrients that are harmful for aquatic ecosystems . Based on its ability of treating water effluents, M. oleifera has become an alternative for the improvement of public health in socially neglected communities.
M. oleifera is considered an eco-friendly plant for its important applications in socio-environmental issues. This plant is resistant to drought and can be cultivated in low-quality soils, causing little alterations in the nutritional components of its different parts. In this context, M. oleifera is a promising tree for several applications, such as battling malnourishment and hunger and providing accessibility to therapeutical resources of social relevance. Besides social and medical applications, M. oleifera oil can be used to sustainably produce a high quality biodiese. Therefore, M. oleifera is a sustainable resource for biotechnology, animal farming, medical sciences, and food industry, as it has mainly been cultivated as human and animal food source.
7. Leading-role of M. oleifera for strengthening
traditional medicine in rural communities
Traditional medicine has been practiced by 80% of the world population, especially in developing countries [108], and its practice is responsible for 90% of the pharmacological
Acknowledgments
This work was supported by grants from the National
Council for Scientific and Technological Development CNPq; Brazil; Processes 307606/2013-9; 443167/2014-1) and Coordination Office for the Improvement of Higher Education Personnel (AEI-0052- 000650100/11).
discoveries in the world. This practice relates
knowledge and beliefs to plants with medicinal properties, based on regional traditions for the alternative treatment of diseases, providing population with greater accessibility to treatment and well-being, especially for those that are devoid of proper public health conditions. Some studies have shown that publicizing and appropriating the practices of traditional medicine by multidisciplinary health teams may reduce the social losses associated with the lack of public policies to promote health, especially for the socially neglected population
In this perspective, M. oleifera reinforces the option of using alternative medicine in disease control, as the plant adapts to the most inhospitable climatic conditions of the poor regions of the world . In the semiarid regions of Brazil, for instance, M. oleifera is well adapted and can be part of the alternative vegetable groups cultivated for the improvement of health in communities where poverty persists, with limited access to drinking water and unavailable public health resources. Moreover, M. oleifera is a relevant food source for the natural nutrition of the tropics that provides health benefits, as source of proteins, essential minerals and antioxidants , hence, it becomes a powerful strategy to battle global malnourishment, especially among children and lactating mothers . Recent studies reveal that the consumption of M. oleifera leaves with acidulated fruit sauces improves the bioavailability of iron and zinc, representing a cheap solution for the deficiency of these ions in the diet of socially neglected population . Therefore, considering the multiple uses of M. oleifera, it is known as the miraculous tree [5,14].
Concerning the ability of M. oleifera to improve water quality and promote the cure of several diseases, including the neglected diseases of the tropics, the great social contribution of this tree is evident, as it promotes the improvement of life quality of the socially neglected population that does not have access to public health, by finding solutions for treating and/or preventing their diseases with traditional medicine. In this context, the encouragement for the cultivation of M. oleifera trees in rural communities may bring benefits to local health and reduce the expenditure with the treatment of neglected diseases, or, as they are popularly known, diseases of poverty.
8. Final considerations
This review summarized the recent research advances for the use and applications of M. oleifera extracts in different areas of biosciences, demonstrating the versatility of this plant. Based on the scientific reports, M. oleifera is an inexpensive, eco-friendly and socially beneficial alternative, especially for the socially neglected population, suffering from poverty and malnutrition and for those who have limited access to technological resources.

References
- El Sohaimy SA, Hamad GM, Mohamed SE, Amar MH, AlHindi RR. Biochemical and functional properties of oringa oleifera leaves and their potential as a functional food. Glob Adv Res J Agric Sci 2015; 4(4): 188-199.
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees life J 2005; 1(5): 1-15.
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phyther Res 2007; 21(1): 17- 25.
- Palada MC. Moringa (Moringa oleifera Lam.): a versatile tree crop with horticultural potential in the subtropical United States. Hort Sci 1996; 31(5): 794-797.
- Fuglie LJ. The miracle tree: the multiple attributes of Moringa. USA: CTA; 2001.
- Lim TK. Moringa oleifera. In: Lim TK, editor. Edible medicinal and non-medicinal plants. Fruits. 1st ed., Vol. 3. London: Springer; 2012, p. 453-485.
- Okuda T, Baes AU, Nishijima W, Okada M. Isolation and characterization of coagulant extracted from oringa oleifera seed by salt solution. Water Res 2001; 35(2): 405-410.
- Chuang P-H, Lee C-W, Chou J-Y, Murugan M, Shieh B-J, Chen H-M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol 2007; 98(1): 232- 236.
- Sanchez-Martín J, Ghebremichael K, Beltr ´ an-Heredia J. Com- ´ parison of single-step and two-step purified coagulants from Moringa oleifera seed for turbidity and DOC removal. Bioresour Technol 2010; 101(15): 6259-6261.
- Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, et al. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol 2009; 47(6): 1109-1116.
- Mbikay M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Front Pharmacol 2012; 3: 24.
- Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003; 64(1): 3-19.
- Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crops Prod 2013; 44: 566-5
- Guevara AP, Vargas C, Sakurai H, Fujiwara Y, Hashimoto K, Maoka T, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res Toxicol Environ Mutagen 1999; 440(2): 181-188.
- Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L, et al. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J Agric Food Chem 2003; 51(12): 3546-3553.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995; 38(4): 957-963.
- Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J Med Food 2010; 13(3): 710-716.
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem 2003; 51(8): 2144-2155.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AH Isolation and structure elucidation of novel hypotensive agents niazinin A, niazinin B, niazimicin and niaziminin A+ B from Moringa oleifera: the first naturally occurring thiocarbamates. J Chem Soc Perkin Trans 1992; 23: 3237-3241.
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 2009; 64(4): 303-311.
- Toppo R, Roy BK, Gora RH, Baxla SL, Kumar P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Vet World 2015; 8(4): 537-540.
- Sharifudin SA, Fakurazi S, Hidayat MT, Hairuszah I, Aris Mohd Moklas M, Arulselvan P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm Biol 2013; 51(3): 279-288.
- Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed Wistar rats. J Ethnopharmacol 2000; 69(1): 21-25.
- Kumari DJ. Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 diabetes mellitus. Bioscan 2010; 5(20): 211-214.
- Gupta A, Gautam MK, Singh RK, Kumar MV, Rao CV,
Goel RK, et al. Immunomodulatory effect of Moringa oleifera
Lam. extract on cyclophosphamide induced toxicity in mice. Indian J Exp Biol 2010; 48(1): 1157-1160. Goel RK, et al. Immunomodulatory effect of Moringa oleifera Lam. extract on cyclophosphamide induced toxicity in mice. Indian J Exp Biol 2010; 48(1): 1157-1160. - Ouédraogo M, Lamien-Sanou A, Ramdé N, Ouédraogo AS, Ouédraogo M, Zongo SP, et al. Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits. Exp Toxicol Pathol 2013; 65(3): 335-339.
- Sutalangka C, Wattanathorn J, Muchimapura S, Thukhammee W. Moringa oleifera mitigates memory impairment and neurodegeneration in animal model of age-related dementia. Oxid Med Cell Longev 2013; 2013: 1-9.
- Kirisattayakul W, Wattanathorn J, Tong-Un T, Muchimapura S, Wannanon P, Jittiwat J. Cerebroprotective effect of Moringa oleifera against focal ischemic stroke induced by middle cerebral artery occlusion. Oxid Med Cell Longev 2013; 2013: 1-10.
- Hannan MA, Kang J-Y, Mohibbullah M, Hong Y-K, Lee H,
Choi J-S, et al. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J Ethnopharmacol 2014; 152(1): 142-150. Choi J-S, et al. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J Ethnopharmacol 2014; 152(1): 142-150. - Mahajan SG, Banerjee A, Chauhan BF, Padh H, Nivsarkar M, Mehta AA. Inhibitory effect of n-butanol fraction of Moringa oleifera Lam. seeds on ovalbumin-induced airway inflammation in a Guinea pig model of asthma. Int J Toxicol 2009; 28(6): 519- 527.
- Nikkon F, Saud ZA, Rahman MH, Haque ME. In vitro antimicrobial activity of the compound isolated from chloroform extract of Moringa oleifera Lam. Pak J Biol Sci 2003; 6(22): 1888-1890.
- Ruckmani K, Kavimani S, Anandan R, Jaykar B. Effect of
Moringa oleifera Lam on paracetamol-induced hepatotoxicity. Indian J Pharm Sci 1998; 60(1): 33-35. Moringa oleifera Lam on paracetamol-induced hepatotoxicity. Indian J Pharm Sci 1998; 60(1): 33-35. - Rocha MFG, Aguiar FLN, Brilhante RSN, Cordeiro RA,
Teixeira CEC, Castelo-Branco DSC, et al. Moringa oleifera and
Vernonia sp. extracts against Candida albicans and Microsporum canis isolates from dogs and cats and analysis of toxicity to Artemia sp. Cienc Rural 2011; 41(10): 1807-1812. Teixeira CEC, Castelo-Branco DSC, et al. Moringa oleifera and Vernonia sp. extracts against Candida albicans and Microsporum canis isolates from dogs and cats and analysis of toxicity to Artemia sp. Cienc Rural 2011; 41(10): 1807-1812. - Cheenpracha S, Park EJ, Yoshida WY, Barit C, Wall M,
Pezzuto JM, et al. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg Med Chem 2010; 18(17): 6598-6602. Pezzuto JM, et al. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg Med Chem 2010; 18(17): 6598-6602. - Mehta K, Balaraman R, Amin AH, Bafna PA, Gulati OD. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol 2003; 86(2): 191-195.
- Agrawal B, Mehta A. Antiasthmatic activity of Moringa oleifera Lam: a clinical study. Indian J Pharmacol 2008; 40(1): 28-31.
- Bukar A, Uba A, Oyeyi T. Antimicrobial profile of Moringa oleifera Lam. extracts against some food-borne microorganisms. Bayero J Pure Appl Sci 2010; 3(1): 43-48.
- Rocha MFG, Alencar LP, Brilhante RSN, Sales JA, Ponte YB, Rodrigues PHA, et al. Moringa oleifera inhibits growth of
Candida spp. and Hortaea werneckii isolated from Macrobrachium amazonicum prawn farming with a wide margin of safety. Cienc Rural 2014; 44(12): 2197-2203 Candida spp. and Hortaea werneckii isolated from Macrobrachium amazonicum prawn farming with a wide margin of safety. Cienc Rural 2014; 44(12): 2197-2203 - Rao RR, George M, Pandalai KM. Pterygospermin; the antibacterial principle of Moringa pterygosperma, Gaertn. Nature 1946; 158: 745-746.
- Das BR, Kurup PA, Narasimha Rao PL. Antibiotic principle from Moringa pterygosperma. VII. Antibacterial activity and chemical structure of compounds related to pterygospermin. Indian J Med Res 1954; 45(2): 191-196.
- Mensah JK, Ikhajiagbe B, Edema NE, Emokhor J. Phytochemical, nutritional and antibacterial properties of dried leaf powder of Moringa oleifera (Lam.) from Edo Central Province, Nigeria. J Nat Prod Plant Resour 2012; 2(1): 107-112.
- Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 2013; 3(8): 623-627.
- Singh RSG, Negi PS, Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Foods 2013; 5(4): 1883-1891.
- Moyo B, Masika PJ, Hugo A, Muchenje V. Nutritional characterization of moringa (Moringa oleifera Lam.) leaves. Afr J Biotechnol 2011; 10(60): 12925-12933.
- Makkar HPS, Becker K. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim Feed Sci Technol 1996; 63(1): 211-228.
- Richter N, Siddhuraju P, Becker K. Evaluation of nutritional quality of Moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture 2003; 217(1): 599-611.
- Oduro I, Ellis WO, Owusu D. Nutritional potential of two leafy vegetables: Moringa oleifera and Ipomoea batatas leaves. Sci Res Essays 2008; 3(2): 57-60.
- Ferreira PMP, Farias DF, Oliveira JT de A, Carvalho A de FU. Moringa oleifera: bioactive compounds and nutritional potential. Rev Nutr 2008; 21(4): 431-437.
- Aslam M, Anwar F, Nadeem R, Rashid U, Kazi TG, Nadeem M. Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab, Pakistan. Asian J Plant Sci 2005; 4: 417-421.
- Abdulkarim SM, Long K, Lai OM, Muhammad SKS, Ghazali HM. Some physico-chemical properties of Moringaoleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem 2005; 93(2): 253-263.
- Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S,
Morales NP, Phivthong-ngam L, et al. The in vitro and ex vivo
antioxidant properties, hypolipidaemic and tiatherosclerotic
activities of water extract of Moringa oleifera Lam. leaves.
J Ethnopharmacol 2008; 116(3): 439-446. - Waterman C, Cheng DM, Rojas-Silva P, Poulev A, Dreifus J, Lila MA, et al. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014; 103: 114-122.
- Charoensin S. Antioxidant and anticancer activities of Moringa oleifera leaves. J Med Plants Res 2014; 8(7): 318-325.
- Purwal L, Pathak AK, Jain UK. In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacol Online 2010; 1(1): 655-665.
- Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, AlShahrani H, Islam M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS One 2015; 10(8). e0135814.
- Elgamily H, Moussa A, Elboraey A, EL-Sayed H, AlMoghazy M, Abdalla A. Microbiological assessment of Moringa oleifera extracts and its incorporation in novel dental remedies against some oral pathogens. Open Access Maced J Med Sci 2016; 4(4): 585-590.
- Nambiar VS, Guin P, Parnami S, Daniel M. Impact of antioxidants from drumstick leaves on the lipid profile of hyperlipidemics. J Herb Med Toxicol 2010; 4(1): 165-172.
- Das N, Sikder K, Ghosh S, Fromenty B, Dey S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J Exp Biol 2012; 50(1): 404-412.
- Caceres A, Saravia A, Rizzo S, Zabala L, De Leon E, Nave F. Pharmacologie properties of Moringa oleifera. 2: screening for antispasmodic, antiinflammatory and diuretic activity. J Ethnopharmacol 1992; 36(3): 233-237.
- Mawouma S, Ponka R, Mbofung CM. Acceptability and solubility of iron and zinc contents of modified Moringa oleifera sauces consumed in the Far-north region of Cameron. Food Sci Nutr 2016; 5(2): 344-348.
- Kushwaha S, Chawla P, Khurana DS. Effect of supplementation of drumstick (Moringa oleifera) and amaranth (Amaranthus tricolor) leaves powder on lipid profile in postmenopausal women. Int J Sci Res Publ 2012; 2(11): 162-168.
- Peixoto JRO, Silva GC, Costa RA, Vieira GHF, Fonteles Filho AA, dos Fernandes Vieira RHS. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med 2011; 4(3): 201-204. Filho AA, dos Fernandes Vieira RHS. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med 2011; 4(3): 201-204
- Peixoto JRO, Silva GC, Costa RA, Vieira GHF, Fonteles Filho AA, dos Fernandes Vieira RHS. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med 2011; 4(3): 201-204. Filho AA, dos Fernandes Vieira RHS. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med 2011; 4(3): 201-204
- Sayeed MA, Hossain MS, Chowdhury MEH, Haque M. In vitro antimicrobial activity of methanolic extract of Moringa oleifera Lam. fruits. J Pharmacogn Phytochem 2012; 1(4): 94-98.
- Onsare JG, Kaur H, Arora DS. Antimicrobial activity of Moringa oleifera from different locations against some human pathogens. Acad J Med Plants 2013; 1(5): 80-91.
- Arora DS, Onsare JG. In vitro antimicrobial evaluation and phytoconstituents of Moringa oleifera pod husks. Ind Crop Prod 2014; 52: 125-135.
- Oluduro AO. Evaluation of antimicrobial properties and nutritional potentials of Moringa oleifera Lam. leaf in South-Western Nigeria. Malays J Microbiol 2012; 8(2): 59-67.
- Rahman MM, Sheikh MMI, Sharmin SA, Islam MS,
Rahman MA, Rahman MM, et al. Antibacterial activity of leaf
juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J Nat Sci 2009; 8(2): 219. Rahman MA, Rahman MM, et al. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J Nat Sci 2009; 8(2): 219. - Rahman MS, Zerin L, Anwar MN. Antibacterial and antifungal activity of Moringa oleifera stem bark. Chittagong Univ J Biol Sci 2008; 3(1–2): 109-117.
- Idris A, Abubakar U. Phytochemical and antibacterial investigations of moringa (Moringa oleifera) leaf extract on selected bacterial pathogens. J Microbiol Antimicrob 2016; 8(5): 28-33.
- Austin B. Vibrios as casual agentes of zoonoses. Vet Microbiol 2010; 140(3–4): 310-317.
- Suarez M, Entenza JM, Doerries C, Meyer E, Bourquin L,
Sutherland J, et al. Expression of a plant-derived peptide
harboring water-cleaning and antimicrobial activities. Biotechnol Bioeng 2003; 81(1): 13-20. Sutherland J, et al. Expression of a plant-derived peptide harboring water-cleaning and antimicrobial activities. Biotechnol Bioeng 2003; 81(1): 13-20. - Sharma A, Patel VK, Ramteke P. Identification of vibriocidal compounds from medicinal plants using chromatographic fingerprinting. World J Microbiol Biotechnol 2009; 25(1): 19-25.
- Padla EP, Solis LT, Levida RM, Shen C-C, Ragasa CY. Antimicrobial isothiocyanates from the seeds of Moringa oleifera Lam. Z Naturforsch C 2012; 67(11–12): 557-564.
- Ndhlala AR, Mulaudzi R, Ncube B, Abdelgadir HA, du
Plooy CP, Van Staden J. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 2014; 19(7): 10480-10494. - Plooy CP, Van Staden J. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 2014; 19(7): 10480-10494. Plooy CP, Van Staden J. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 2014; 19(7): 10480-10494.
- Jabeen R, Shahid M, Jamil A, Ashraf M. Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera. Pak J Bot 2008; 40(4): 1349-1358.
- Raj AJ, Gopalakrishnan VK, Yadav SA, Dorairaj S. Antimicrobial activity of Moringa oleifera (Lam.) root extract. J Pharm Res 2011; 4(5): 1426-1430
- Ishnava KB, Chauhan KH, Bhatt CA. Screening of antifungal activity of various plant leaves extracts from Indian plants. Arch Phytopathol Plant Prot 2012; 45(2): 152-160.
- Marrufo T, Nazzaro F, Mancini E, Fratianni F, Coppola R, De Martino L, et al. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. cultivated in Mozambique. Molecules 2013; 18(9): 10989-11000.
- Onsare JG, Arora DS. Antibiofilm potential of flavonoids
extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol 2015; 118(2): 313-325.
extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol 2015; 118(2): 313-325. - Lipipun V, Kurokawa M, Suttisri R, Taweechotipatr P,
Pramyothin P, Hattori M, et al. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antivir Res 2003; 60(3): 175-180. - Waiyaput W, Payungporn S, Issara-Amphorn J, Panjaworayan T. Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells. BMC Complement Altern Med 2012; 6(12): 246.
- Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res 2015; 114(9): 3315-3325.
- Prabhu K, Murugan K, Nareshkumar A, Ramasubramanian N, Bragadeeswaran S. Larvicidal and repellent potential of Moringa oleifera against malarial vector, Anopheles stephensi Liston (Insecta: Diptera: Culicidae). Asian Pac J Trop Biomed 2011; 1(2): 124-129.
- Agra-Neto AC, Napoleão TH, Pontual EV, de Lima Santos ND, de Andrade Luz L, de Oliveira CMF, et al. Effect of Moringa oleifera lectins on survival and enzyme activities of Aedes aegypti larvae susceptible and resistant to organophosphate. Parasitol Res 2014; 113(1): 175-184.
- Ali GH, Elitaweel GE, Ali MA. The cytotoxicity and antimicrobial efficiency of Moringa oleifera seeds extracts. Int J Environ Stud 2004; 61(6): 699-708.
- Ferreira PM, Carvalho AF, Farias DF, Cariolano NG, Melo VM, Queiroz MG, et al. Larvicidal activity of the water extract of Moringa oleifera seeds against Aedes aegypti and its toxicity upon laboratory animals. An Acad Bras Cienc 2009; 81(2): 207- 216.
- Mangale SM, Chonde SG, Raut PD. Use of Moringa oleifera (drumstick) seed as natural absorbent and an antimicrobial agent for ground water treatment. Res J Recent Sci 2012; 1(3): 31-40.
- Rocha-Filho CA, Albuquerque LP, Silva LR, Silva PC,
Coelho LC, Navarro DM, et al. Assessment of toxicity of Moringa oleifera flower extract to Biomphalaria glabrata, Schistosoma mansoni and Artemia salina. Chemosphere 2015; 132: 188- 192. - Salazar-Gamez LL, Luna-delRisco M, Cano RE. Comparative study between M. oleifera and aluminum sulfate for water treatment: case study Colombia. Environ Monit Assess 2015; 187(10): 668.
- Santos TR, Silva MF, Nishi L, Vieira AM, Klein MR,
Andrade MB, et al. Development of a magnetic coagulant based on Moringa oleifera seed extract for water treatment. Environ Sci Pollut Res Int 2016; 23(8): 7692-7700. - Ghebremichael K, Gebremedhin N, Amy G. Performance of Moringa oleifera as a biosorbent for chromium removal. Water Sci Technol 2010; 62(5): 1106-1111.
- Almeida IL, Filho NR, Alves MI, Carvalho BG, Coelho NM.
Removal of BTEX from aqueous solution using Moringa oleifera seed cake. Environ Technol (United Kingdom) 2012; 33(11): 1299-1305. - Shebek K, Schantz AB, Sines I, Lauser K, Velegol S, Kumar M. The flocculating cationic polypeptide from Moringa oleifera seeds damages bacterial cell membranes by causing membrane fusion. Langmuir 2015; 31(15): 4496-4502.
- Reddy DH, Seshaiah K, Reddy AV, Rao MM, Wang MC. Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: equilibrium and kinetic studies. J Hazard Mater 2010; 174(1): 831-838.
- Makkar HP, Francis G, Becker K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Anim Feed Sci Technol 2007; 1(9): 1371-1391.
- Oluduro OA, Aderiye BI, Connolly JD, Akintayo ET, Famurewa O. Characterization and antimicrobial activity of 4-(bD-glucopyranosyl-1/4-a-L-rhamnopyranosyloxy)-benzyl thiocarboxamide; a novel bioactive compound from Moringa oleifera seed extract. Folia Microbiol (Praha) 2010; 55(5): 422-426.
- Ferreira RS, Napoleão TH, Santos AF, Sa RA, Carneiro-da- Cunha MG, Morais MM, et al. Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera. Lett Appl Microbiol 2011; 53(2): 186-192.
- Viera GH, Mourão JA, Angelo AM, Costa RA, Vieira RHSF. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev Inst Med Trop Sao Paulo 2010; 52(3): 129-132.
- Brilhante RS, Sales JA, de Souza Sampaio CM, Barbosa FG, de Araújo Neto Paiva M, de Melo Guedes GM, et al. Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts. Asian Pac J Trop Med 2015; 8(11): 919-922.
- Ramos SCS, de Oliveira JCS, da C^amara CAG, Castelar I, Carvalho AFFU, Lima-Filho JV. Antibacterial and cytotoxic
properties of some plant crude extracts used in Northeastern folk medicine. Rev Bras Farmacogn 2009; 19(2A): 376-381. - Bijina B, Chellappan S, Krishna JG, Basheer SM, Elyas KK, Bahkali AH, et al. Protease inhibitor from Moringa oleifera with potential for use as therapeutic drug and as seafood preservative. Saudi J Biol Sci 2011; 18(3): 273-281.
- Freitas JH, de Santana KV, do Nascimento AC, de Paiva SC, de Moura MC, Coelho LC, et al. Evaluation of using aluminum sulfate and water-soluble Moringa oleifera seed lectin to reduce turbidity and toxicity of polluted stream water. Chemosphere 2016; 163: 133-141.
- Tavares FO, Pinto LA, Basseti FJ, Vieira MF, Bergamasco R, Vieira AM. Environmentally friendly biosorbents (husks, pods and seeds) from Moringa oleifera for Pb(II) removal from contaminated water. Environ Technol 2017; 17: 1-11.
- Gade WK, Buchberger SG, Wendell D, Kupferle MJ. Application of Moringa oleifera seed extract to treat coffee fermentation wastewater. J Hazard Mater 2017; 17(329): 102-109.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis 2016; 15(1): 212.
- Moshi MJ. Current and future prospects of integrating traditional and alternative medicine in the management of diseases in Tanzania. Tanzan J Health Res 2005; 7(3): 159-167.
- Fokunang CN, Ndikum V, Tabi OY, Jiofack RB, Ngameni B, Guedje NM, et al. Traditional medicine: past, present and future research and development prospects and integration in the national health system of Cameroon. Afr J Tradit Complement Altern Med 2011; 8(3): 284-295.
- Dansi A, Vodouhe R, Azokpota P, Yedomonhan H, Assogba P, Adjatin A, et al. Diversity of the neglected and underutilized crop species of importance in Benin. Sci World J 2012; 2012: 932-947.
- Vandebrok I. Intercultural health and ethnobotany: how to improve healthcare for underserved and minority communities? J Ethnopharmacol 2013; 148(3): 746-754.
- Razis AFA, Ibrahim MD, Kntayya SB. Health benefits of Moriga oleifera. Asian Pac J Cancer Prev 2014; 15(20): 8571-8576.
Credit : ScienceDirect