How Functional Is Moringa oleifera? A Review of Its Nutritive, Medicinal, and Socioeconomic Potential
Oluwadara Oluwaseun Alegbeleye1
Abstract
Moringa oleifera is an important tropical food plant that seems to have great nutritional, therapeutic, industrial, agricultural, and socioeconomic value. Dietary consumption of its parts and preparations is encouraged by several organizations, health food enthusiasts, and other specialists as a strategy of personal health preservation and self-medication in the treatment of various diseases. Studies extoling its ability to mitigate various degenerative ailments now exist in both the scientific and the popular literature. At face value, and considering the volume of reports available, much of this enthusiasm seems to be indeed justified. However, it is imperative to distinguish rigorous scientific evidence from anecdote. To achieve this, relevant experimental and review articles were sought and read critically to identify recent patterns and trends on this subject matter. Studies on the medicinal and functional properties of M. oleifera are available from various parts of the world, especially developing regions. Attempts have been made to parse the contemporary scientific data available supporting the claims regarding the phytochemical, nutritive, medicinal, environmental, agricultural, and socioeconomic capabilities of this plant. Studies reviewed provide compelling, albeit preliminary experimental evidence of therapeutic potential of the plant. It is important that M. oleifera products and preparations be properly chemically characterized and standardized before being administered.
Keywords
Global South, Moringa oleifera, functional food, bioactive compounds, nutraceuticals
Introduction
Moringa oleifera Lamarack is a fast growing, perennial angiosperm tree that may grow as high as 7 to 15 m and reach a diameter of 20 to 40 cm at chest height.1,2 It belongs to the Moringaceae family and is generally regarded as a vegetable, a medicinal plant, and a source of cooking oil in the developing world. It is indigenous to the subHimalayan tracts of India, Pakistan, Bangladesh, and Afghanistan where it is known by various regional names such as benzolive, kelor,
drumstick tree, horseradish tree, marango, and malunggay. Recently, it has garnered medical and socioeconomic attention in the tropics and
Department of Food Science, Faculty of Food Engineering, The University of Campinas, Sao Paulo, Brazil
Corresponding Author:
Oluwadara Oluwaseun Alegbeleye, Department of Food
Science, University of Campinas, Sa˜o Paulo, Brazil.
Email: seunalegbeleye@gmail.com

Figure 1. Moringa oleifera tree growing on a stack of granite stones.
subtropics such as in Western, Eastern, and Southern Africa; tropical Asia; Latin America; the Caribbean; and the Pacific Islands where it is now being widely cultivated and has naturalized. It is an easily cultivated tree, famous for its low
demand for soil nutrients and water thereby capable of withstanding destitute soils and drought (illustrated in Figure 1). It tolerates a wide range of rainfall, with minimum annual rainfall requirements estimated at 250 mm and maximum at over 3000 mm.3 It is an important food plant highly nutritious and generally esteemed as a functional food with all parts established to be edible.4-6 A wide variety of nutritional, prophylactic, and therapeutic virtues have been attributed to its roots, bark, leaves, flowers, sap, fruits, and seeds. There is some documented evidence that M. oleifera possesses medicinal attributes, including hypotensive, hypoglycemic, anticancer, radioprotective, thyroid hormone regulatory, ntiobesity, antipyretic, antiepileptic, and diuretic attributes among others.7-11 Numerous traditional and scientific studies
have been conducted on the attributes of M. oleifera, the output of which have been the subject of many extensive reviews.1,2,4,6-8 Many experts have proposed the use of M. oleifera as a complementary medical option or for use in relief and prevention of disease symptoms. However, in
spite of these studies, reviews, recommendations, and widespread claims, Western (conventional) medicine has been spectacularly hesitant in exploring its nutritional and medicinal potential. This lukewarm attitude is curious, as other
“superfoods” such as garlic and green tea have enjoyed better reception. However, the recent “healthy eating” trend where many consumers are inclined to consume wholesome foods, are skeptical of synthetic medications, as well as the advancement of “green” medicine and so on, has placed a spotlight on M. oleifera among other “healthy” options. This enthusiasm has generated significant international hype and commercial boom, and it is therefore necessary to assess the
strength of evidence and determine whether firm conclusions regarding the safety and efficacy of M. oleifera can be inferred from the available studies.
This review is a timely assessment of the
research progress achieved regarding the potential of this versatile plant. Here, the exceptional bioactive, free radical, and heavy metal scavenging potentials of M. oleifera are discussed. The nutritional properties, its potential use in food
processing, medicinal attributes, as well as overall socioeconomic relevance are considered. The possibilities of harnessing its value to combat malnutrition, improve health and well-being,
detoxify domestic and industrial wastewaters, develop the food industry for local and international markets, and create jobs in urban and rural *centers are explored.
Procedures for Literature Search and Analysis
Peer-reviewed medical and nutrition science articles were sought and reviewed for this article. Electronic databases (PubMed, Scopus, Web of Science, and Google Scholar) were explored. The search was conducted using M. oleifera, drumstick, horseradish, malunggay, kelor, nebeday, benzolive, and saijhan within the title of the article. Articles on other Moringa varieties other than oleifera as well as articles in which the word “drumstick” pertained to other topics such as medical condition or transcription were not included. Original research and review articles as well as nonpeer-reviewed articles including newspaper articles, technical reports, conference proceedings, and so on, were critically read. Peer-reviewed articles presenting data/information on the therapeutic, nutritional, agricultural, biodiesel, and socioeconomic potential of M. oleifera were analyzed for inclusion in this review. The non-peer-reviewed sources were analyzed to achieve in-depth understanding of the subject matter.
Phytochemistry of Moringa oleifera
Moringa oleifera is rich in bioactive phytochemicals that confer many of the health benefits on it.4,12 Moringa has abundant deposits of compounds containing simple sugar, rhamnose as
well as a somewhat distinctive group of compounds called glucosinolates and isothiocyanates.4 These compounds are believed to possess hypotensive, chemopreventive, and antibacterial activity because compounds such as 4- (4’-O-acetyl-a-L-rhamnopyranosyloxy)benzyl isothiocyanate, 4-(a-L-rhamnopyranosyloxy) benzyl isothiocyanate, niazimicin,13 pterygospermin,14 benzyl isothiocyanate,15 and 4-(a-L-rhamnopyranosyloxy)benzyl glucosinolate4 have been identified in studies from which hypotensive, anticancer, and antibacterial activities of M
oleifera were inferred. Flavonoids and phenolics are other structural classes of phytochemicals that have been identified from M. oleifera. Flavonoids and phenolic acids are collectively referred to as phenolic compounds.1 Quercetin and kaempferol, in their 3-O-glycoside forms, are the predominant
flavonols in M. oleifera leaves.1 Quercetin, a potent antioxidant, exhibiting multiple therapeutic properties is found in high concentrations in M. oleifera leaves.3 It is usually found occurring as quercetin-3-O-b-d-glucoside also known as isoquercitrin or isotrifolin and has been shown to have antidyslipidemic, hypotensive, and antidiabetic effects in the obese Zucker rat.16 It has also been found to reduce hyperlipidemia and atherosclerosis in High-carbohydrate-diet (HCD) or high-fat diet (HFD) rabbits.1 Another very important chemical compound that has been identified in Moringa is chlorogenic acid, which has been shown to aid in glucose metabolism in rats. Studies in rats have demonstrated its ability to inhibit glucose-6-phosphate translocase in rat liver, thereby reducing hepatic gluconeogenesis and glycogenolysis.17,18 The stem bark has been reported to contain alkaloids, moringine and moringinine,3 which have been associated with improved glucose tolerance,19 as well as vanillin, b-sitostenone, 4-hydroxymellin, and octacosanoic acid. Niaziminin, which is believed to possess hypotensive properties, has been extracted and described from the M. oleifera leaves. The leaves also contain phytosterols such as b-sitosterol, which have the potential to reduce intestinal uptake of dietary cholesterol.20,21 It contains other well-known phytochemicals including carotenoids (b-carotene), flavonoids, and pro-vitamin A.1,4 Some of the structures of common bioactive compounds isolated from M. oleifera are shown in Figure 2.
Nutritional Properties of Moringa oleifera
Moringa oleifera serves as an extremely valuable food source. Its use in human nutrition as well as in development of balanced diets in animal nutrition has been justified by a few studies.22-25 It is reportedly an excellent indigenous source of highly digestible protein, calcium, iron,
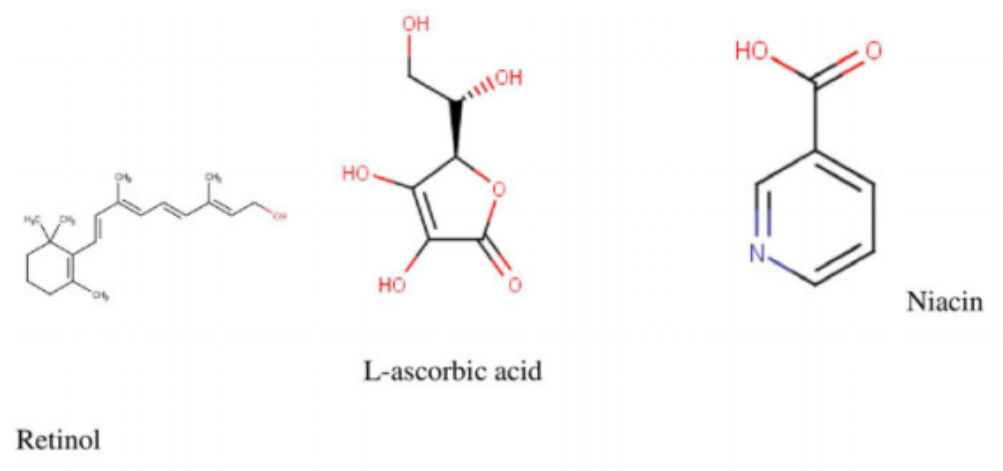
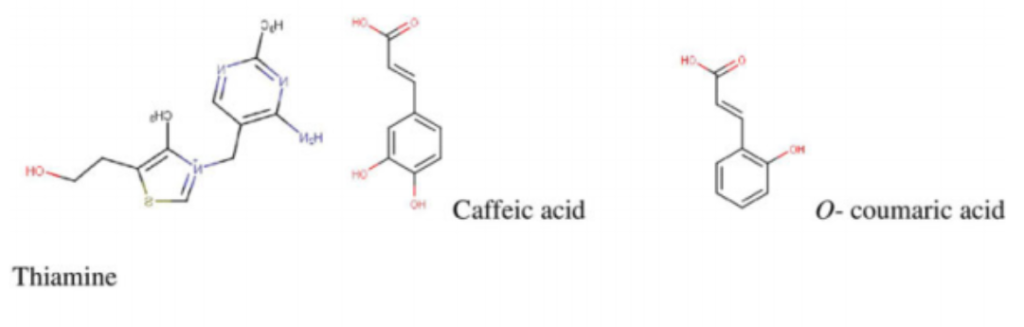
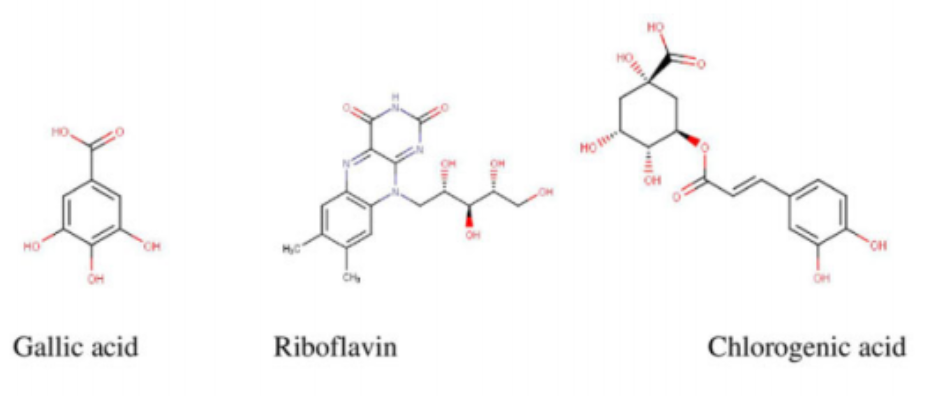
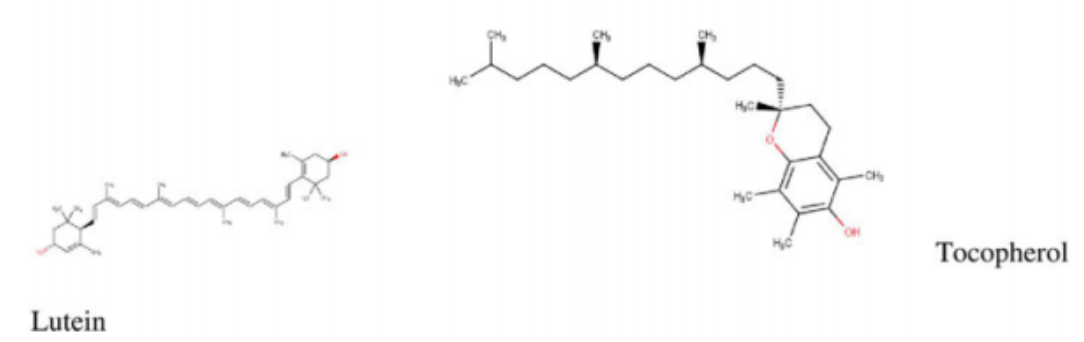
potassium, vitamins, trace metal ions, essential amino acids, antioxidants, and carotenoids suitable for combating malnutrition in many developing nations of the world where malnourishment is a major concern.26-33 It contains arginine and histidine—2 amino acids that are especially relevant for infant nutrition.7 A study by Valdez-Solana et al34 evaluated the chemical composition and nutritional values of dried M. oleifera leaf powder obtained in Mexico. The proximate analysis showed that M. oleifera leaves are a good source of fiber, proteins, lipids, carbohydrates, calcium, potassium, and magnesium. Phenolics (gallic acid as well as chlorogenic acid)
and flavonols (rutin, luteolin, quercetin, apigenin, and kaempferol), which are potent natural antioxidants, were also isolated. In addition, the high ash content isolated from the dried M. oleifera leaves indicate that the leaves are a good source of inorganic minerals. A similar study by Sa´nchez-Machado et al,35 which assessed the biochemical properties of M. oleifera obtained from the North-Western Mexico state of Sonora, reported an abundant deposit of protein, ash, lipids, fatty acids, amino acids, and dietary fibers from the leaves, immature pods, and flowers of M. oleifera. Moyo et al32 determined the nutritional value of Moringa leaves using proximate and Van Soest methods. The dried leaves had crude protein levels as high as 30.3% and 19 amino acids. In addition, they had calcium, phosphorus, magnesium, potassium, sodium, sulfur, zinc, copper, manganese, iron, and selenium. Seventeen fatty acids were detected including a-linolenic, heneicosanoic, -linolenic, palmitic, and capric acid among others. Vitamins, b-carotene, fibers, tannins, and polyphenols were also identified.
Another important factor that makes M. oleifera nutritionally relevant is that it is drought resistant, fast growing, easy to cultivate and manage, and is capable of adapting in virtually all tropical and subtropical climates.3 Furthermore, Moringa leaves production peaks at the end of dry season when fruits and vegetables are typically in short supply and may only be found in irrigated agroecosystems. Moringa oleifera thus serves as an alternative source of good nutrition at this time of year, being a tremendous source of vitamins,
minerals, and antioxidants as well as offering a balanced supply of amino acids.
The leaves are rich in a wide repertoire of vitamins and minerals. According to the Trees for Life Organization, ounce for ounce, Moringa leaves contain more vitamin A than carrots, more calcium than milk, more iron than spinach, more vitamin C than oranges, and more potassium than bananas. Furthermore, the protein quality of Moringa leaves rivals that of milk and eggs. The leaves and other parts of the tree contain high amounts of crude protein and amino acids, thus serving as an outstanding source of plant protein for vegans and vegetarians.36-38 Moringa oleifera also serves as an important source of essential fatty acids, which are required for optimal cellular health. Nongovernmental organizations, Trees for Life, Church World Service and Educational Concerns for Hunger Organization, have enthusiastically advocated Moringa as “Natural nutrition for the Tropics.” Alternative Action for African Development and Church World Service assessed the capability of Moringa leaf powder to prevent or cure malnutrition in pregnant and nursing mothers and their children in Southwestern Senegal in 1997/1998. Malnutrition was a serious challenge in this area, at the time, with more than 600 malnourished infants receiving medical treatment yearly. During the assessment, doctors, nurses, and midwives were trained to prepare and use Moringa leaf powder to treat malnutrition. Women in the rural settlements were also trained
to prepare and use Moringa to fortify foods. Weight increase and improved overall health were observed at the end of the assessment. Pregnant women fully recovered from anemia and delivered babies with higher birth weights.39 An increase in lactation was also observed, verifying claims that M. oleifera is a galactagogue as in the Philippines where it has been dubbed “Mother’s best friend” due to its ability to improve lactating mothers’ milk production.3,38,40,41 Its lactogenic properties have been recognized,42,43 and increased serum prolactin levels have been observed in association with intake ofMoringa preparations/supplements.42 In Philippines, for example, the
consumption of M. oleifera is widespread, but expecting and lactating mothers have reported improved milk production and increased appetites
Depending on the guidelines designed by the World Health Organization (based on weight-forlength Z score), a child can be classified as normal, mildly, or moderately malnourished. In
severe cases of malnutrition, physiological anomalies such as infections, impaired liver, and intestinal function as well as electrolyte imbalances are likely to have set in. Due to these physiological abnormalities, Moringa oleifera is not a recommended regimen in this case. A severely malnourished child cannot tolerate iron or normal amounts of dietary proteins, fats, and sodium. The child needs to recover from this emergency state by intensive hospital care, where infections
are treated and new ones are prevented, electrolyte balance is restored, and intensive 24-hour feeding regimen is instituted.39 Therefore, until the child’s condition is stable and appetite returns, the diet should be rich in carbohydrates; contain potassium, magnesium, and other essential minerals; but should be low in protein, fat, and sodium; and completely lacking in iron supplements. Moringa leaves, which are high in iron and protein content, are thus not suitable for use at this stage (the initial treatment of the severely malnourished).
According to the report by Fuglie, mild or moderate malnutrition, which is not at the terminal stage and is less critical, can be treated using M. oleifera. The physiological abnormalities are much less severe, and a balanced diet containing all essential nutrients in the right proportions is required for full recovery.39 In Senegal and other parts of West Africa, adding Moringa on a daily basis to a child’s meal has demonstrated tremendous ability to bring about rapid recovery from moderate malnutrition. More importantly, successfully treating malnutrition is good, but preventing it is even better. Due to Moringa’s rich nutrient profile, it can serve as an excellent resource to prevent malnourishment.
Moringa oleifera as a Food Additive
There is mounting evidence that incorporating M. oleifera into food and food products improves the physicochemical and organoleptic characteristics and also extends the shelf life of food. Various parts of the Moringa tree have exhibited
antimicrobial activity.3,44-46 Food-borne diseases are rife in many parts of the world, particularly the developing world. The toll in terms of finances, human health, and suffering is enormous. There is some research evidence that M. oleifera may play a relevant role in the prevention of food-borne diseases. Moringa seed and leaf extracts have exhibited antimicrobial properties that inhibit bacterial growth.32,47,48 A study by Bukar et al49 illustrated that M. oleifera ethanol leaves extract exhibited broad-spectrum activity against food-borne pathogens: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterobacter aerogenes. The same study demonstrated that M. oleifera seed chloroform extract showed antimicrobial activity against E coli, Salmonella typhimurium, Mucor, and Rhizopus species. The study illustrated that M. oleifera is capable of inhibiting the growth of certain food-borne pathogens as well as some spoilage microorganisms in food under laboratory experimental conditions. Another study by Walter et al50 demonstrated the ability of M. oleifera to inhibit the growth of E coli, Sa typhi, and Vibrio cholerae. Isothiocyanates from M. oleifera exhibited antibiotic activity against Helicobacter pylori.4 This pathogen is prominent in poor, medically underserved regions of the world; is a major cause of gastritis, gastric, and duodenal ulcers; and is a major risk factor for gastric cancer. Further research is desirable to explore the potential of this activity on the treatment of human H pylori infection.
Antibiofilm potential of M. oleifera has been documented, and this is an important research prospect.51 A recent study by Lee et al52 demonstrated the ability of M. oleifera liquid extracts to inhibit biofilm formation by a pathogen S aureus. Biofilm formation by pathogens plays crucial roles in their persistence and antibiotic resistance. Biofilms are microbial colonies that form when single microorganisms attach and aggregate on a hydrated surface and undergo a “lifestyle switch,” giving up life as a single cell to live on a surface in an adhesive cell matrix with other microorganisms.53 They are usually resistant to antimicrobial agents, and studies have revealed that cells within a particular biofilm are usually of diverse community properties.
Moringa oleifera extends the shelf life of lipidcontaining foods due to the presence of various types of antioxidant compounds such as ascorbic acid, flavonoids, phenolics, and carotenoids.54 Certain preparations may be used to preserve meat and other food products from oxidative deterioration.55,56 Oxidative stress in farm animals and lipid–protein oxidation in meat and meat products may be prevented by incorporating Moringa into animal nutrition and using Moringa extracts to fortify meat products.56 A study by Moyo et al57 demonstrated the antioxidative potential of M.
oleifera. The study investigated the antioxidative effects of M. oleifera supplement on the activities of superoxide dismutase (SOD), catalase (CAT), lipid peroxidation (LPO), and glutathione (GSH) in goats. Both acetone and aqueous M. oleifera leaf extracts were determined to have potent antioxidant activities, increasing the antioxidant activity of GSH, SOD, and catalase in the goats studied. The study attributed the antioxidative potential to the presence of polyphenolic compounds in the M. oleifera leaves. Nadeem et al58 showed that M. oleifera oil improved the oxidative stability of butter oil. It improved the oxidative stability, conferred higher resistance toward shoot up of peroxide value at elevated temperature, anisidine value, and formation of oxidation products. The oil was also observed to improve the nutritional value of the butter oil by increasing the concentration of oleic acid.
There is some research evidence suggesting that M. oleifera could be used to boost the organoleptic qualities of food and food products such as pastries and meat among others. Research by Dachana et al59 has demonstrated the effects of dried Moringa leaves on the rheological, microstructural, nutritional, and the overall quality characteristics of cookies. It was observed that the addition of dried Moringa leaves increased fairnograph water absorption and decreased dough stability, amylograph pasting temperature as well as peak viscosity. It also increased dough hardness, decreased cohesiveness, and spread ratio of the cookies. Furthermore, protein, iron, calcium, b-carotene as well as dietary fiber contents of the cookies increased by the end of the experiments. Scholars such as Hazra et al60 treated cooked ground buffalo meat with aqueous
solution of crude extract of M. oleifera leaves. This significantly improved meat pH and water holding capacity and lowered cooking loss and thiobarbituric acid value. It also improved the
quality of the meat by enhancing the tenderness, juiciness, and preventing discoloration as well as off-flavor formation. Microbial load in terms of total plate count was found to be decreased significantly in treated samples, also demonstrating the antimicrobial activity of M. oleifera. In a fairly recent study, Manaois et al61 incorporated fresh and powdered M. oleifera into rice crackers to serve as a dietary supplement. Not only did M. oleifera significantly improve the b-carotene, vitamin C, and calcium content, the Moringa-rice crackers developed were shelf stable for up to 3 weeks.
Medicinal Use
A number of curative, pharmacological, and prophylactic properties have been ascribed to various parts of this highly esteemed tree.62 Claims abound about the disease prevention and treatment benefits derived from both the dietary and the topical administration of M. oleifera. According to Ayurvedic traditional medicine practitioners, M. oleifera can prevent and cure about 300 diseases.63 It is capable of acting as cardiac and circulatory stimulants and possess antitumor, antipyretic, antiepileptic, anti-inflammatory, antiulcer, antispasmodic, diuretic, antihypertensive, hypoglycemic, cholesterol lowering, antioxidant, antibacterial,
antifungal, antidiabetic, anti-asthmatic as well as hepatoprotective virtues.6,10,62,64 It has been and continues to be used by folk medicine practitioners to prevent, mitigate, or treat many ailments and diseases. It is also used as alternative medicine in home remedy preparations in many parts of the world for treatment and management of allergies, inflammation, and diseases.65,66 In many parts of the developing world, it is widely consumed for
self-medication by patients affected by diabetes, hypertension, and HIV/AIDS.38,67-70 In spite of these widespread claims, however, data obtained from human studies are limited, and many of the available studies are inconclusive with standardization being a major issue. Some medicinal benefits of M. oleifera tree preparations are shown in
Table 1.
Table 1. Medicinal and Biological Properties of Moringa oleifera Plant Extracts/Preparations.
Moringa Plant Part/Extract
or Preparation63
Phytochemical/Biological/Medicinal Activity
References
Fresh leaves
Rich source of vitamins A, B1, B2, and B3 (Thiamine, Riboflavin, Niacin) and b-carotene.
Serves as a purgative, used to treat piles, fevers, sore throat, bronchitis, eye and ear infections, scurvy, and catarrh. Also serves as antiulcer, anti-inflammatory, diuretic and is used for wound treatment. Leaf
extracts (and preparations) exhibit antioxidant, antitumor,
antimicrobial, antidiabetic and antidyslipidemic activities (aqueous, hydroalcohol or alcohol). Leaf extracts exhibit a wide range of biological activities including tissue protective (liver, kidneys, heart, testes, and lungs) activities, analgesic, antiulcer, antihypertensive, radioprotective as well as immunomodulatory actions
1,6,63,68,71-74
Dried leaves
Contains thiamine, riboflavin, niacin as well as vitamins C and E (ascorbic acid and tocopherol). Also contains b carotene and lutein, polyphenols, caffeic acid, chlorogenic acid, o-coumaric acid, ellagic acid, ferulic acid, gallic acid, flavonoids, epicatechin, genistein, isorhamnetin, kaempferol, myricetin, quercetin, saponins, phytates rutin, 4-hydroxybenzyl, 4-a-L-rhamnopyranosyloxy)-benzyl, 4-O- (a-L-acetylrhamnopyranosyloxy)-benzyl
63
Seed
Contains alkaloids, reducing sugars, saponins, proteins, flavonoids, and phenolic compounds.
Has hepatoprotective, antipyretic, antimicrobial, and antihypertensive potential
3,75,76
Stem bark
Used to treat ulcers and used as a pain killer to relieve tooth ache. It contains antioxidants and phenolic compounds. Also exhibits antitubercular and antimicrobial activity
66,77,78
Root
Used as a laxative, possesses antimicrobial, antispasmodic, antiinflammatory, antilithic, vesicant, and hepatoprotective activities
66,72
Gum
Serves as an astringent and rubefacient. Contains L-arabinose, Dgalactose, D-glucuronic acid, L-rhamnose, D-mannose, D-xylose, and leucoanthocyanin
79,80
Flower
Hepatoprotective, antibacterial, and fungicidal activities, antiinflammatory, stimulant, aphrodisiac
76,81,82
Methanolic flower extract
Tannins, saponin, flavonoids, mycertin, terpenoids, reducing sugars, alkaloids, anthraquinones
63
Aqueous extract of foliage
Hypolipidemic and antiatherosclerotic, oxidative DNA damage protective activity, downregulation of nuclear factor kB, immunity against Herpes Simplex virus Type 1 (HSV-1)
55,83,84
Ethanol extract of foliage
Upregulation of TNF-a, antihyperglycemic, and hypolipidemic activity
85,86
Methanol extract of foliage
Antioxidant, anti-inflammatory, and antinociceptive
84
Aqueous leaves extract
Anticancer activity, flavonoids, steroids, terpenoids, and scavenging of radicals
54,87
Ethanol extract of leaves
Hypoglycemic activity
88
Seed methanolic extracts
Bioinsecticidal property
89
Seed oil
Contains vitamin A, b-carotene, and precursor of vitamin A
15,90
Antihyperglycemic and Antidyslipidemic
Properties of Moringa oleifera
Some preliminary trials have attempted to demonstrate M. oleifera’s potential in the treatment of
hyperglycemia and dyslipidemia, primarily in people with type 2 diabetes.67,91 Research evidence points to its ability to regulate blood sugar levels using laboratory animals, particularly rats.7
Ndong et al92 demonstrated that M. oleifera leaf powder decreased blood glucose levels by 23% compared to controls after administering 2 g of leaf powder per kilogram to rats. Another study by Jaiswal et al71 showed that M. oleifera leaf aqueous extracts decreased blood glucose levels in a dose-dependent manner using doses of 100 to 300 mg/kg. Investigation by Tende et al88 assessed the effects of ethanol extracts of M. oleifera leaves on the blood glucose levels of streptozotocin (STE)-induced diabetic rats. The study administered the extract at doses of 250 and 500 mg/kg intraperitoneally. A significant reduction in blood glucose levels was observed in fasted STE-induced diabetic rats but not in control, normotensive animals. This effect was attributed to the terpenoid content of the extract, although there is no direct evidence to support this contention. A study by Gupta et al93 demonstrated that M. oleifera is capable of preventing STE-induced diabetes. The study showed that the progression of diabetes was significantly reduced after M. oleifera was administered to STEinduced diabetic albino rats. Moringa oleifera induced a significant reduction in serum glucose and nitric oxide, with a concomitant increase in serum insulin and protein levels. It increased antioxidant levels in pancreatic tissue, accompanied by reduction in levels of thiobarbituric acidreactive substances. The treatment also significantly reversed the histoarchitectural damage to the islet cells. Experimentation by Yassa and Tohamy94 assessed the antidiabetic and antioxidative potential of M. oleifera aqueous leaf extracts in STE-induced diabetic rats. Moringa oleifera treatment significantly reduced fasting blood plasma glucose (380%-145%), increased reduced glutathione (22%-73%), and decreased malondialdehyde (385%-186%) in contrast to control levels. Reversal of damage of islet cells was also observed subsequent to M. oleifera leaf extract administration. The antihyperglycemic effects of the aqueous leaf extract could be attributed in part to the presence of an intestinal sucrose inhibitor,96 although this action does not effectively explain the effect of the leaf extract in response to glucose tolerance test. Ghasi et al97 examined the antidyslipidemic effects of M. oleifera leaves on rats fed an HFD. Wistar rats were
fed an HFD containing 16% (w/w) fat, with or without an aqueous extract of M. oleifera leaves at a daily dose of 1 g/kg/bw for 30 days. In untreated animals, the diet induced a 30% increase in plasma total cholesterol. In treated rats, the increase was reduced to 14%.
There are a few documented human studies demonstrating sugar regulatory properties of M. oleifera. William et al91 examined how the addition of M. oleifera to a standardized meal taken after an overnight fast affected the 1- and 2-hour postprandial glucose (PPG) levels, relative to the standard meal alone or a 75 g oral glucose load in patients with untreated type 2 diabetes. It was a controlled study, and M. oleifera was compared to bitter gourd (Momordica charantia) and curry leaves (Murraya koenigii). Compared to the glucose load, standard meals with or without herbal supplements induced a significantly lower rise in PPG as derived from area under the curve (AUC) values. However, when the fortified meals were compared to standard meals, only the M. oleifera–fortified meal elicited a lower response (21%; P < .01). Plasma insulin AUC values did not differ significantly between the 2 meals, suggesting that the hypoglycemic effect of M. oleifera leaf supplementation was not due to increased insulin secretion.
Kumari et al98 evaluated the hypoglycemic effect of M. oleifera leaf dietary consumption in patients with type 2 diabetes aged 30 to 60 years over a 40-day period. Forty-six patients were involved in this experiment: 32 men and 14 women. These patients were not receiving treatment for hyperglycemia. There was a control group of 9 patients: 4 men and 5 women. The experimental group was fed a dose of 8 g of M. oleifera leaf powder daily. Fasting plasma glucose (FPG) and PPG at the end of the experiments were compared to baseline levels. Final values did not differ significantly from baseline in the control group. They were, however, significantly
reduced in the experimental group (FPG: 28%, P < .01; PPG: 26%, P < .05). Kushwaha et al99 documented a study that assessed 30 postmenopausal women who were supplemented daily with 7 g of M. oleifera leaf powder for 3 months. A control group also consisted of 30 postmenopausal women. Results showed a significant
reduction in fasting blood glucose levels (13.5%) as well as an increase in hemoglobin (17.5%). An increase in serum glutathione peroxidase (18.0%), superoxide dismutase (10.4%), and ascorbic acid (44.4%), with a concomitant decrease in malondialdehyde (16.3%; LPO) markers of antioxidant properties was also observed. The study did not report any adverse effects.
Hepatoprotective Properties of Moringa oleifera
Certain studies have explored the efficacy of M. oleifera in the treatment of liver diseases.100-102 A study conducted by Hamza et al76 indicated that Moringa possesses anti-inflammatory properties against carbon tetrachloride-induced liver damage and fibrosis. This finding was confirmed by the decrease in globulin levels in serum and the myeloperoxidase activity in liver. Sreelatha and Padma101 used M. oleifera leaves extract in an in vitro system involving rat liver slices. This was
observed to weaken the toxicity of carbon tetrachloride evidenced by a decrease in LPO and increase in the antioxidant enzymes: glutathione peroxidase, glutathione reductase, catalase, SOD, and glutathione S-transferase (GST). Another study by Das et al102 showed that aqueous extract of M. oleifera leaves prevented liver damage in mice fed with a high-fat diet. This was demonstrated by reductions in tissue histopathology and serum activities of marker enzymes: aspartate amino transferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) as well as reduced LPO and increases in reduced glutathione. There is some experimental evidence that M. oleifera may protect against hepatotoxic effects of certain drugs. Pari and Kumar103 showed that ethanol extracts of M. oleifera leaves
protected rats against the hepatotoxicity of antitubercular drugs such as isoniazid, rifampicin, and pyrazinamide. The extract decreased druginduced levels of AST, ALT, and ALP as well as bilirubin and inhibited drug-induced LPO in the liver. Various studies have demonstrated that M. oleifera leaf extracts have the capacity to prevent liver toxicity induced by acetaminophen (Paracetamol).104-106 Fakurazi et al104 showed that aqueous ethanol extracts of M. oleifera
(200 and 800 mg/kg) prevented acetaminopheninduced liver damage evidenced by reduction in AST, ALT, and ALP as well as increase in hepatic glutathione. Sharifudin et al107 also described the capability of hydroethanol extracts of M. oleifera leaves and flowers at doses of 200 and 400 mg/kg administered intraperitoneally to inhibit acetaminophen-induced hepatotoxicity.
Al-Said et al108 evaluated the antioxidant, antioxidative stress, and hepatoprotective activity of Ben oil (Moringa seed oil, known as Ben oil is a sweet, nonsticky oil, which is rich in oleic acid, tocopherols as well as sterols and resists rancidity)109 against carbon tetrachloride-induced LPO
and hepatic damage in rats. Elevated serum enzymatic (Glutamic-oxaloacetic transaminase [GOT], Glutamic-pyruvic-transaminase [GPT], ALP, and Gamma-glutamyl-transpeptidase [GGT]) and bilirubin levels normalized after 21 days of orally administering the oil. This indicates that Ben oil is
capable of preserving the structural integrity of the hepatocellular membrane and inhibits liver cell damage induced by CCl4. This deduction was confirmed by histopathological studies. Also, a significant depletion in the level of malondialdehyde and an improvement in non-protein sulfhydryl (NP-SH) and total protein (TP) contents in the liver tissue were observed.
The Use of Moringa oleifera in Cancer Therapy
Use of M. oleifera in treatment of various cancers is being explored.87,110-112 Experimental evidence indicates that M. oleifera can protect biological cells from the oxidative DNA damage associated with cancer and degenerative diseases.63,113 Furthermore, the presence of bioactive compounds, 4-(4’-O-acetyl-alpha-Lrhamnopyranosyloxy), benzyl isothiocyanate, b-sitosterol-3-O-b-d-glucopyranoside, and niazimicin which have been shown to be potent inhibitors of phorbol ester in lymphoblastoid cells, has made it highly relevant in cancer therapy.62,114 Also, its potential to induce apoptosis suggests that it could effectively hinder tumor progression.115 Guevara et al114 used bioactive compounds (Isothiocyanate rhamnoside, niazimicin, and sitosterol glucoside)
obtained from the ethanol extract of M. oleifera seeds. The antitumor potential of the isolated compounds was assessed using an in vitro assay that examined their inhibitory effects on EpsteinBarr virus early antigen (EBV-EA) activation in Raji cells induced by the tumor promoter 12-Otetradecanoyl-phorbol-13-acetate (TPA). All the isolates tested showed satisfactory inhibitory effects on the induction of EBV-EA activation without significant cytotoxicity on Raji cells. The bioactive compounds exhibited potent inhibitory activity in which about 50%, 80 to 90%, and 100% inhibition of activation were observed at 100, 500, and 1000-fold mol ratio to 32 pmol of TPA, respectively, and preserved the high viability of Raji cells even at the highest concentration of 1000 mol ratio/TPA. Other compounds that have been associated with antitumor activity and have been identified in Moringa are b-sitosterol-3-O-bD-glucopyranoside and benzyl iosthiocyanate.4
Sreelatha et al determined the antiproliferative and apoptopic effects of M. oleifera aqueous leaf extracts using human tumor KB cell line as a model system. Qualitative analysis of the leaf extracts showed the presence of phenolic compounds such as quercetin and kaempferol, flavonoids, and trace amounts of alkaloids. The percentage of cell viability was evaluated using MTT assay. The study demonstrated the antiproliferative effect of M. oleifera by showing its ability to induce loss of cell viability, morphology change, internucleosomal DNA fragmentation, and reactive oxygen species generation in KB cells. Tiloke et al116 demonstrated the antiproliferative activity of M. oleifera leaves extract against cancer alveolar epithelial cells. A similar study by Jung87 showed that aqueous extracts of M. oleifera leaves exhibited significant antineoplastic activity against a lung cancer cell line as well as several other types of cancer cells. The extract induced apoptosis, inhibited tumor cell growth, and lowered the internal levels of reactive oxygen species in human cancer cells. Moringa oleifera roots have exhibited unique estrogenic, antiestrogenic, progestational, and antiprogestational activities.117 Its effectiveness in treating ovarian cancer became apparent after the publication of recent studies demonstrating that benzyl isothiocyanate and phenethyl
isothiocyanate induce apoptosis in ovarian cancer cells in vitro. Taha et al118 demonstrated the ability of Moringa leaves to ameliorate and protect rats’ bladder from cyclophosphamide (CP)-induced toxicity. Cyclophosphamide is an alkylating antineoplastic agent that is commonly used to treat solid tumors and B-cell malignant disease and has been associated with urinary bladder damage by induced oxidative stress. Another study by Jaiswal et al71 reported a significant increase in the activity of oxidative free radical scavenging enzymes (SOD, CAT, and GST) when the aqueous extract of M. oleifera leaves was administered to normal and diabetic rats using in vivo and in vitro assays. A concomitant decrease in LPO was also observed, demonstrating the antioxidant activity
of Moringa. The study concluded that regular dietary intake of M. oleifera leaves may protect normal as well as diabetic people against oxidative damage.
Moringa oleifera may be capable of ameliorating heavy metal toxicity.119 Gupta et al120 investigated the therapeutic efficacy of oral administration of M. oleifera seed powder on arsenic poisoning in rats. The study showed that treatment of postarsenic exposure using the seed powder of M. oleifera significantly protected the rats against the general toxic effects of arsenic and exhibited antioxidative potential in vitro and in vivo against hydroxyl radicals generated by Fenton reaction. It brought about a moderate but significant depletion in arsenic from blood and tissues of the animals. The study also documents that the seed powder exhibited chelating properties against arsenic toxicity. Sasikala et al121 described the ability of M. oleifera leaf extracts to prevent cataractogenesis induced by selenite poisoning in rat
pups (Sprague-Dawley strain) weighing 10 to 12 g by the eighth day of the experiment. Sodium selenite (4 mg/g) was administered subcutaneously to the rats on the 10th day to induce cataract. Some rats received an additional 2.5 mg/g of the extracts from the 8th day through the 15th day. By the 16th
day, cataracts were visualized. Moringa oleifera extract significantly prevented morphological changes and oxidative damage to the lens. It effectively prevented cataractogenesis in the seleniteinfected rats by enhancing the activities of
antioxidant enzyme, reducing the intensity of LPO and inhibiting free radical generation. Sadek122 reported that ethanol extract of M. oleifera leaves protected against chromium-induced testicular activity in rats. The extract was administered orally on a daily basis (500 mg/kg) for 60 days
to rats that were spiked with 8 mg potassium chromate intraperitoneally. The ethanol extract significantly ameliorated the testicular chromium effects on sperm parameters, local immunity, inflammatory markers, and antioxidant enzyme activities. A recent study by Oliveira et al123 demonstrated the
ability of Moringa oleifera husks to biosorb heavy metals in chicken feeds in Brazil.
Other Medicinal Attributes
Certain studies have explored the antiulcer potentials of M. oleifera. According to Chattopadhyay et al,119 M. oleifera possesses valuable antiulcer, antisecretory, and cytoprotective activity. Their study used an ethanolic root bark extract of M. oleifera to determine its potential in antiulcer drug formation. Moringa oleifera significantly reduced the free acidity, total acidity, the ulcer index as well as increased the pH of the gastric content in albino Wistar rats.
It has been suggested that some preparations/ extracts may prevent cardiovascular diseases. Nandave et al124 demonstrated the cardioprotective effects of lyophilized hydroalcoholic extracts of M. oleifera. The study used Wistar albino male rats in the isoproterenol-induced model of myocardial infarction. Chronic M. oleifera treatment resulted in significant favorable modulation of the biochemical enzymes (SOD, CAT, glutathione peroxidase, lactate dehydrogenase, and creatine kinase-MB) and significantly prevented the rise in LPO in myocardial tissue. The study attributed the significant cardioprotective effect to M. oleifera’s antioxidant, antiperoxidative, and myocardial preservative properties. There are claims asserting that Moringa leaf juice can effectively stabilize blood pressure. Substances such as nitrile, mustard oil glycosides, and thiocarbamate glycosides, which have been associated with stabilizing blood pressure, have been isolated from Moringa leaves.
Socioeconomic Relevance of Moringa oleifera
In addition to its nutritional and medicinal attributes, M. oleifera possesses many other intriguing qualities, thereby serving aesthetic, agricultural, and industrial purposes. It is an ornamental plant and is therefore used to beautify yards and home fronts, sometimes serving as a fence in many parts of the developing world such as in Northern Nigeria.127 Like many other trees, the trees serve as windbreaks and reduce soil erosion. It is used in lumber production as well as for light construction work in many parts of the developing world. The
coarse fiber is often used for the production of ropes or mats. It is also used for contaminant flocculation as well as water purification.4,63,128-132
Moringa oleifera has great potential to urify domestic water sources as well as to be used in agricultural, industrial, and municipal wastewater treatment. A study by Ferreira et al133 demonstrated the natural biocoagulant properties of M.
oleifera for water. The study showed that M. oleifera reduced turbidity, suspended solids and bacteria, and may be used on a commercial scale for wastewater treatment. Other studies134-140 have shown that M. oleifera is an effective, nontoxic, environmentally friendly, low cost, sustainable means of purifying water, aesthetically and microbiologically. It may, therefore, serve as a substitute for synthetic (usually imported) purification agents (which may not be as biodegradable and environmentally friendly as M. oleifera), thus reducing expenditure by developing countries and encouraging local trade. It, therefore, has economic, public health, and environmental sustainability relevance in this regard. Its potential to detoxify industrial wastewaters has received some attention in recent time. It seems possible to use certain M. oleifera treatments to remove organics from wastewaters. For example, its pods have been demonstrated to be suitable sorbents for the removal of organics such as benzene, toluene, ethylbenzene, and cumene. Since its pods, seeds, and leaves are cheap, indigenous, and readily available, this offers a costeffective alternative for wastewater treatment. Its relevant use in agriculture has been documented and is currently being explored in many
parts of the developing world.48 Due to its coagulation, flocculation, and sedimentation properties, it has impressive capacity to detoxify agricultural wastes. In intensive animal production systems such as aquaculture, Moringa has great potential to replace chemicals and antibiotics for waste treatment and disease prevention and control, since it is an abundant source of antioxidants, antimicrobials, and coagulating substances.
Crude, ethanol, and aqueous extracts of M. oleifera have been demonstrated to reduce microbial levels in fish and shrimp farming.3,142,143 Moringa oleifera seed extracts have exhibited antimicrobial activity against human pathogens such as S aureus, E coli, and V cholerae isolated from Tilapia (Oreochromis niloticus) and shrimp (Litopenaeus vannamei). Furthermore, ethanol extracts of pods, seeds, and leaves as well as chloroform extracts from flowers have exhibited antibiotic potential against microorganisms such as Vibrio spp., Hortaea wernecki, and Candida spp. recovered from prawns (Macrobrachium amazonicum).
The antimicrobial potential of M. oleifera against aquaculture relevant microbes is worth exploring further because of its economic potential, as many of them are potentially zoonotic, opportunistic pathogens that typically trigger economic losses in aquaculture as well as pose public health threats.145 Moreover, besides the inhibitory effects of M. oleifera on different agricultural/human relevant microbes, it has also been reported that crude extracts of M. oleifera leaves and seeds also inhibit microbial protease activity, which causes muscular degradation in seafood such as fish and shrimp during storage. It is therefore possible to use these extracts/preparations to control seafood proteolysis and deterioration during low temperature storage. In animal husbandry, the leaves and twigs serve as fodder/forage for livestock, and local farmers usually introduce these into droughtridden agricultural areas to augment fodder supply.4 Its use as a natural plant growth enhancer has been described.63 The leaves are rich in zeatin (a plant hormone belonging to the cytokinin group), and the leaf extracts have been used to stimulate plant growth, thereby increasing crop
yield. Research has shown its successful use with wheat, rice, and maize.63,146 Its leaf and seed extracts exhibit biopesticide activity and seem to have the capacity to control the larvae, pupa, and adults of Anopheles stephensi and Trogoderma granarium. 63,89 The extracts may also reduce the incidence of fungal species on groundnut seeds.146
In many rural areas, it is used as a fuel source, for example, firewood for cooking and charcoal production. One contemporary application of Moringa (seeds) is as biomass for biofuel production. With the current urgency to develop alternative (cleaner) sources of energy generation, biodiesel has gained a lot of attention among
other viable options. Biodiesel is a renewable and ecofriendly alternative to conventional nonrenewable fossil fuel sources. Biodiesels are usually manufactured by chemically reacting lipids
of vegetable oil and animal fat.84 Cottonseed, palm, peanut, rapeseed, soybean, and sunflower are common examples of sources of vegetable oils that have been successfully used in biodiesel production. However, the use of these is limited by global food insecurity, price, and availability.84 Therefore, over the last several years, less conventional oils such as Jatropha, Moringa, Pongamia, and Tobacco have been the subject of research in this regard.84 It has been established that fatty acid methyl esters of M. oleifera seed oil satisfy all the main criteria of biodiesel standards
of Germany, other parts of Europe, and the United States.84,147 Moringa seeds have sufficiently high oil content (about 40%) as well as high-quality fatty acid composition (oleic acid > 70%).63 In addition, the oil wields significant resistance against oxidative degradation, thus making it a good candidate for biodiesel production after transesterification.63 Another benefit of utilizing M. oleifera for biofuel production is that it does not directly compete with available farmland and food crops, being a second-generation
fuel production process and can be cultivated under suboptimal conditions. It is therefore an acceptable substitute to fossil fuels, even compared with biodiesel derived from other plant varieties.
Some reports suggest the leaves are a useful domestic cleaning agent. It is also used i the production of cosmetics and health-care products such as body and hair moisturizers, perfume, conditioners, lotions, ointments, and so on.63 Ben oil is commonly used in the preparation of some salads and as a lubricant.
In many parts of the developed world, Moringa-based nutritional supplements, teas, herbal infusions, condiments such as curry, beverages, food products, and baby formula are being marketed. Although the efficacy of M. oleifera is widely known in the developing world, there are not many industrial applications of its
parts yet, particularly in Africa. In other parts of the world—the United States, China and Europe—the number of industrial, pharmaceutical, medical, and food-processing patents and trademarks are growing. It will be impressive to see this sort of advancements in developing countries
as well. It is in fact important that the developing world participate in this Moringa commercial boom. The plant is indigenous to this region and can serve numerous health, nutritional, and economic purposes.
Due to mainstream beliefs regarding its nutritive and medicinal efficacy, demand for Moringa plant parts and products has increased in recent times. There are now more Moringa plantations and processing (although more commonly, small scale) outfits that are employing labor and enabling people financially. These economic benefits are not restricted to rural areas. In Nigeria, for instance, many urban dwellers grow Moringa in their yards and sell the pods, seeds, and leaves to interested consumers.127,150 So many others ingeniously process the leaves, for example, by drying or grounding and then distribute and sell. Some incorporate these preparations into locally produced cosmetic products. It thus serves as a source of income and employment opportunities for both rural and urban dwellers.
Final Remarks
Based on the facts gleaned from the reviewed studies, M. oleifera apparently has great potential to improve nutrition among poor households and boost overall well-being of humans in the developing world and beyond. It appears to be a very important plant with enormous potentials yet to
be fully explored in food science. However, it is rapidly receiving some international attention. For example, the Food and Agricultural Organization of the United Nations recognized Moringa as the September 2014 traditional crop of the month. Its remarkable relevance in combating malnutrition has been abundantly highlighted in the literature.
A balanced varied diet rich in meat, root, grains, fruits, and vegetables is necessary for optimum nutrition and well-being. However, a significant proportion of the world’s population cannot
afford such variety consistently. Some vital food sources are seasonally unavailable, and during this lean period, many people including infants consume meals that are deficient in vital nutrients. In addition to this, however, malnutrition is due to numerous other factors such as, pervasive illiteracy, poverty, famine, disease pathogens, and suboptimal drinking water. Numerous approaches have been proposed and explored to combat malnutrition. Many of these focus on rectifying micronutrient deficiencies alone, and many of them have not effectively eradicated malnutrition because these approaches and programs did not address other related problems. However, compared to programs that rely on imported products and foreign donor support and subsidies, Moringa is advantageous because it is a local resource. Its use is, therefore, bound to be cost effective and sustainable.
The antimicrobial virtues of M. oleifera can play relevant roles in minimizing the incidence of water- and food-borne diseases such as dysentery, diarrhea, and cholera, especially in rural communities. Some of the studies reviewed also indicate its potential as raw material for industries, its use in food preservation, and so on. Due to the myriad biochemical attributes of this plant,
it can serve as a pivotal part in designing sustainable community development approaches.
Conclusions
Overall, M. oleifera is a valuable food crop whose nutritive, healing, and socioeconomic potentials are apparent. Numerous studies have described its ability to regulate physiological processes as well
as prevent and cure diseases. Its ability to heal a
myriad of chronic afflictions is becoming more evident. A plethora of traditional medicine references attest to its curative potential and scientific validation of these claims is rapidly developing to substantiate some of the assertions. Scientists, researchers, and conventional medicine are beginning to acknowledge the relevance of the tree.
However, many of the available claims have not been validated by placebo-controlled, randomized clinical trials and have not been published in high visibility journals. Many of the studies reviewed in this summary, particularly those on the medicinal benefits of the tree, require further validation to corroborate their findings. Further scientific research and more rigorous clinical studies are required. There are so many shortfalls
and gaps in the available science, for instance, many of the available studies have been conducted using rats, and in as much as we can extrapolate from these, more human studies are highly desirable. Also, standardization is a serious issue, as it seems that different extracts from different parts of the tree exert unique therapeutic effects. It is difficult to ascertain what parts offer the best
benefits and the effects that extraction methods have on the potency of preparations. Furthermore, dosage is still a major challenge in this field of research. It is very important to standardize research before adopting parts or preparations of M. oleifera as a supplement or curative measure. There is very limited research regarding the
potential pharmaceutical side effects, allergies, contraindications, and drug interactions with prescribed medicines. Other problems include inconsistent methodologies as well as insufficient rigor
and detail in experiments. Finally, balanced evidence from complementary and alternative medicine is required in order to offer M. oleifera use the credibility it seemingly deserves.
Acknowledgments
The author gratefully acknowledges the financial support of Conselho Nacional de Desenvolvimento Cientifico e Tecnolo´gico (CNPq).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Conselho Nacional de Desenvolvimento Cientifico e Tecnolo´gico (CNPq) (award grant number 148279/2017 -1).
ORCID iD
Oluwadara Oluwaseun Alegbeleye http://orcid.org/ 0000-0002-2708-1301
References
- Mbikay M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Front Pharmacol. 2012;3:1-3.
- Ferreira PMP, Farias DF, Oliveira JT de A, Carvalho A de FU. Moringa oleifera: bioactive compounds and nutritional potential. Rev Nutr. 2008; 21(4):431-437.
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21(1):17-25.
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees for life Journal. 2005;1(5):1-15.
- Cajuday LA, Pocsidio GL. Effects of Moringa oleifera Lam. (Moringaceae) on the reproduction of male mice (Mus musculus). J Med Plants Res. 2010;4(12):1115-1121.
- Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015; 29(6):796-804.
- Fuglie LJ, Service CW, Eng NY (USA), Development AA for A, Eng D (Senegal). The miracle tree: Moringa oleifera, natural nutrition for the tropics. 1999. http://agris.fao.org/agris-search/search.do? recordID¼XF2015018648. Accessed October 17, 2017.
- Paliwal R, Sharma VP. A review on horse radish tree (Moringa oleifera): a multipurpose tree with high economic and commercial importance. Asian J Biotechnol. 2011;3(4):317-328.
- Sharma V, Paliwal DR, Jammeda P, Sharma S. Chemopreventive efficacy of Moringa oleifera pods against 7, 12-dimethylbenz[a]anthracene induced hepatic carcinogenesis in mice. Asian Pac J Cancer Prev. 2012;13(6):2563-2569
- Chen C, Zhang B, Huang Q, Fu X, Liu RH. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: characterization and hypoglycemic activity. Ind Crops Prod. 2017;100(supplement C):1-11.
- Metwally FM, Rashad HM, Ahmed HH, Mahmoud AA, Abdol Raouf ER, Abdalla AM. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac J Trop Biomed. 2017;7(3): 214-21.
- Chumark P, Khunawat P, Sanvarinda Y, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol. 2008;116(3):439-446.
- Akhtar AH, Ahmad KU. Anti-ulcerogenic evaluation of the methanolic extracts of some indigenous medicinal plants of Pakistan in aspirin-ulcerated rats. J Ethnopharmacol. 1995;46(1):1-6.
- Anderson DMW, Bell PC, Gill MCL, McDougall FJ, McNab CGA. The gum exudates from chloroxylon swietenia, sclerocarya caffra, Azadirachta indica and Moringa oleifera. Phytochemistry. 1985;25(1):247-249.
- Anwar F, Bhanger MI. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J Agric Food Chem. 2003; 51(22):6558-6563.
- Rivera L, Moro´n R, Sa´nchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16(9):2081-2087.
- Hemmerle H, Burger HJ, Below P, et al. Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6- phosphate translocase. J Med Chem. 1997;40(2): 137-145.
- Karthikesan K, Pari L, Menon VP. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem Biol Interact. 2010;188(3):643-650.
- Bour S, Visentin V, Pr´evot D, et al. Effects of oral administration of benzylamine on glucose tolerance and lipid metabolism in rats. J Physiol Biochem. 2005;61(2):371-379.
- Jain PG, Patil SD, Haswani NG, Girase MV, Surana SJ. Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Rev Bras Farmacogn. 2010;20(6):969-973
- Lin X, Racette SB, Lefevre M, et al. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: a controlled feeding trial. Eur J Clin Nutr. 2010; 64(12):1481-1487.
- Aregheore EM. Intake and digestibility of Moringa oleifera–batiki grass mixtures by growing goats. Small Rumin Res. 2002;46(1):23-28.
- Richter N, Siddhuraju P, Becker K. Evaluation of nutritional quality of Moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture. 2003;217(1):599-611.
- Mendieta-Araica B, Spo¨rndly R, Reyes-Sa´nchez N, Spo¨rndly E. Moringa (Moringa oleifera) leaf meal as a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livest Sci. 2011;137(1): 10-17.
- Ogbe AO, Affiku JP. Proximate study, mineral and anti-nutrient composition of Moringa oleifera leaves harvested from Lafia, Nigeria: potential benefits in poultry nutrition and health. J Microbiol Biotechnol Food Sci. 2011;1(3):296.
- Bennett RN, Mellon FA, Foidl N, et al. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J Agric Food Chem. 2003; 51(12):3546-3553.
- Aslam M, Anwar F, Nadeem R, Rashid U, Kazi TG, Nadeem M. Mineral Composition of Moringa oleifera Leaves and Pods from Different Regions of Punjab, Pakistan. Asian J Plant Sci. 2005. http:// agris.fao.org/agris-search/search.do? recor dID¼DJ2012050175. Accessed October 17, 2017.
- Manguro LO, Lemmen P. Phenolics of Moringa oleifera leaves. Nat Prod Res. 2007;21(1):56-68. 29. Thurber MD, Fahey JW. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the “diffusion of innovations” theory. Ecol Food Nutr. 2009;48(3):212-225.
- Amaglo NK, Bennett RN, Curto RB, et al. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010; 122(4):1047-1054.
- Gowrishankar R, Kumar M, Menon V, et al. Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera (Moringaceae), and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biol Trace Elem Res. 2010;133(3):357-363.
- Moyo B, Masika PJ, Hugo A, Muchenje V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr J Biotechnol. 2011;10(60): 12925-12933.
- Taha NR, Amin HA, Sultan AA. The protective effect of Moringa oleifera leaves against cyclophosphamide-induced urinary bladder toxicity in rats. Tissue Cell. 2015;47(1):94-104.
- Valdez-Solana MA, Mejı´a-Garcı´a VY, T´ellezValencia A, et al. Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. J Chem. 2015; 28; 2015.
- Sa´nchez-Machado DI, Nu´ n˜ ez-Gast´elum JA, Reyes-Moreno C, Ramı´rez-Wong B, Lo´pez-Cervantes J. Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods. 2010; 3(3):175-180.
- Dhakar R, Maurya S, Pooniya B, Bairwa N, Gupta M, Sanwarmal. Moringa: The herbal gold to combat malnutrition. Chron Young Sci. 2011;2(3): 119-125.
- Kholif AE, Morsy TA, Gouda GA, Anele UY, Galyean ML. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim Feed Sci Technol. 2016;217(suppl C):45-55.
- Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-okeng JW. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res. 2010;4(9):753-757.
- Fuglie LJ 2001. Combating malnutrition with Moringa. Church World Service, Senegal. Scientific Research Publish.http://www.scirp.org/(S(3 51jmbntvnsjt1aadkposzje))/reference/Reference sPapers.aspx? ReferenceID¼1450478. Accessed October 19, 2017.
- Popoola JO, Obembe OO. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J Ethnopharmacol. 2013;150(2):682-691.
- Abe R, Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J Ethnopharmacol. 2013; 145(2):554-565
- Raguindin PFN, Dans LF, King JF. Moringa oleifera as a Galactagogue. Breastfeed Med. 2014; 9(6):323-324.
- Foong SC, Tan ML, Marasco LA, Ho JJ, Foong WC. Oral galactagogues for increasing breast-milk production in mothers of non-hospitalised term infants. In: Cochrane Database of Systematic
- Reviews. John Wiley & Sons, Ltd; 2015. http:// onlinelibrary.wiley.com/doi/10.1002/14651858. CD011505/abstract. Accessed October 24, 2017.
- Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol. 2007;98(1):232-236.
- Pal SK, Mukherjee PK, Saha K, Pal M, Saha BP. Antimicrobial action of the leaf extract of Moringa oleifera lam. Anc Sci Life. 1995;14(3): 197-199.
- Zaffer M, Ahmad S, Sharma R, Mahajan S, Gupta A, Agnihotri RK. Antibacterial activity of bark extracts of Moringa oleifera Lam. against some selected bacteria. Pak J Pharm Sci. 2014;27(6):1857-1862.
- Ezhilarasi AA, Vijaya JJ, Kaviyarasu K, Maaza M, Ayeshamariam A, Kennedy LJ. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. J Photochem Photobiol B. 2016;164:352-360.
- Brilhante RSN, Sales JA, Pereira VS, et al. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac J Trop Med. 2017;10(7):621-630.
- Bukar A, Uba A, Oyeyi T. Antimicrobial profile of Moringa oleifera Lam. Extracts against some food-borne microorganisms. BAJOPAS. 2010; 3(1):43-48.
- Walter A, Samuel W, Peter A, Joseph O. Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases. Afr J Microbiol Res. 2011;5(2):153-157.
- Onsare JG., Arora DS. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol. 2015;118(2):313-325
- Lee JH, Kim YG, Park JG, Lee J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control. 2017;80(suppl C):74-82.
- Lemon KP, Higgins DE, Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J Bacteriol. 2007;189(12):4418-4424.
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51(8):2144-2155.
- Singh BN, Singh BR, Singh RL, et al. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol. 2009;47(6):1109-1116.
- Falowo AB, Fayemi PO, Muchenje V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res Int. 2014;64(suppl C):171-181.
- Moyo B, Oyedemi S, Masika PJ, Muchenje V. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012;91(4):441-447.
- Nadeem M, Abdullah M, Hussain I, Inayat S, Javid A, Zahoor Y. Antioxidant potential of Moringa oleifera leaf extract for the stabilisation of butter at refrigeration temperature. Czech J. Food Sci. 2013;31(4): 332-339.
- Dachana KB., Rajiv J, Indrani D, Prakash J. Effect of dried moringa (Moringa Oleifera Lam) leaves on rheological, microstructural, nutritional, textural and organoleptic characteristics of cookies. J Food Qual. 2010;33(5):660-677.
- Hazra S, Biswas S, Bhattacharyya D, Das SK, Khan A. Quality of cooked ground buffalo meat treated with the crude extracts of Moringa oleifera (Lam.) leaves. J Food Sci Technol. 2012;49(2): 240-245.
- Manaois RV, Morales AV, Abilgos-Ramos RG. Acceptability, shelf life and nutritional quality of Moringa-supplemented rice crackers. PHILIPP J CROP SCI. 2013;38(2):1-8.
- Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci. 2015;16(6):12791-12835.
- Agrawal B, Mehta A. Antiasthmatic activity of Moringa oleifera Lam: A clinical study. Indian J Pharmacol. 2008;40(1):28-31. 65. Iwu MM. Handbook of African medicinal plants, second edition. CRC Press; 2014. 508 p.
- Mootoosamy A, Fawzi Mahomoodally M. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J Ethnopharmacol. 2014;151(1): 413-444.
- Die`ye AM, Sarr A, Diop SN, et al. Medicinal plants and the treatment of diabetes in Senegal: survey with patients. Fundam Clin Pharmacol. 2008;22(2):211-216.
- Monera T, Maponga C. Moringa oleifera supplementation by patients on antiretroviral therapy. J Int AIDS Soc. 2010;13(4):P188.
- Sivasankari B, Anandharaj M, Gunasekaran P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J Ethnopharmacol. 2014;153(2):408-423.
- Yabesh JEM, Prabhu S, Vijayakumar S. An ethnobotanical study of medicinal plants used by traditional healers in silent valley of Kerala, India. J Ethnopharmacol. 2014;154(3):774-789.
- Jaiswal D, Kumar Rai P, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123(3):392-396.
- Ca´ceres A, Saravia A, Rizzo S, Zabala L, De Leon E, Nave F. Pharmacologie properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J Ethnopharmacol. 1992;36(3):233-237.
- Luqman S, Srivastava S, Kumar R, Maurya AK, Chanda D. Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid Based Complement Alternat Med. 2012;2012:519084. http://dx.doi.org/10.1155/ 2012/519084
- Arora DS, Onsare JG. In vitro antimicrobial evaluation and phytoconstituents of Moringa oleifera pod husks. Ind Crops Prod. 2014;52(suppl C): 125-135.
- Abdull Razis AF, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev APJCP. 2014;15(20):8571-8576.
- Unuigbe CA, Okeri HA, Erharuyi O, Oghenero EE, Obamedo DA. Phytochemical and antioxidant evaluation of Moringa oleifera (Moringaceae) leaf and seed. J Pharm Bioresour. 2015;11:51-57.
- Hamza AA. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol. 2010;48(1):345-355.
- Jaiswal D, Rai PK, Mehta S, et al. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med. 2013;6(6): 426-432.
- Kumbhare M, Guleha V, Sivakumar T. Estimation of total phenolic content, cytotoxicity and in–vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac J Trop Dis. 2012;2(2):144-150.
- Bhattacharya SB, Das AK, Banerji N. Chemical investigations on the gum exudate from sajna (Moringa oleifera). Carbohydr Res. 1982;102(1): 253-262.
- Morton JF. The horseradish tree, Moringa pterygosperma (Moringaceae)—A boon to Arid Lands? Econ Bot. 1991;45(3):318-333.
- Ramachandran C, Peter KV, Gopalakrishnan PK. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ Bot. 1980;34(3):276-283.
- Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed. 2013;(8): 623-627.
- Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13(1):212.
- Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6(2):203.
- Akanni OE, Adedeji AL, Oloke KJ. Abstract 3792: Upregulation of TNF-a by ethanol extract of Moringa oleifera leaves in benzene-induced leukemic Wister rat: a possible mechanism of anticancer property. Cancer Res. 2014;74:3792-3792.
- Muhammad HI, Asmawi MZ, Khan NAK. A review on promising phytochemical, nutritional and glycemic control studies on Moringa oleifera Lam. in tropical and sub-tropical regions. Asian Pac J Trop Biomed. 2016;6(10):896-902.
- Jung IL. Soluble Extract from Moringa oleifera Leaves with a New Anticancer Activity. PLOS ONE. 2014;9(4): e95492.
- Tende JA, Ezekiel I, Dikko AA, Goji AD. Effect of ethanolic leaves extract of Moringa oleifera on blood glucose levels of streptozocin-induced diabetics and normoglycemic Wistar rats. Br J Pharmacol. 2011;2(1):1-4.
- Prabhu K, Murugan K, Nareshkumar A, Ramasubramanian N, Bragadeeswaran S. Larvicidal and repellent potential of Moringa oleifera against malarial vector, Anopheles stephensi Liston (Insecta: Diptera: Culicidae). Asian Pac J Trop Biomed. 2011;1(2):124-129.
- Anwar F, Ashraf M, Bhanger MI. Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. J Am Oil Chem Soc. 2005; 82(1):45-51.
- William F, Lakshminarayanan S, Chegu H. Effect of some Indian vegetables on the glucose and insulin response in diabetic subjects. Int. J. Food Sci. Nutr. 1993;44(3):191-195.
- Ndong M, Uehara M, Katsumata SI, Suzuki K. Effects of oral administration of Moringa oleifera Lam on glucose tolerance in Goto-Kakizaki and Wistar rats. J Clin Biochem Nutr. 2007;40(3): 229-233.
- Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabet. 2012;4(2): 164-171.
- Yassa HD, Tohamy AF. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced Diabetes mellitus in adult rats. Acta Histochem. 2014;116(5):844-854.
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. Seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res. Int. 2015
- Adisakwattana S, Chanathong B. Alphaglucosidase inhibitory activity and lipidlowering mechanisms of Moringa oleifera leaf extract. Eur Rev Med Pharmacol Sci. 2011; 15(7):803-808.
- Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed Wistar rats. J Ethnopharmacol. 2000;69(1):21-25.
- Kumari DJ. Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 diabetes mellitus. The BioScan. 2010;5(20): 211-214.
- Kushwaha S, Chawla P, Kochhar A. Effect of supplementation of drumstick (Moringa oleifera) and amaranth (Amaranthus tricolor) leaves powder on antioxidant profile and oxidative status among postmenopausal women. J Food Sci Technol. 2014;51(11):3464-3469.
- Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2009;47(9):2196-2201.
- Sreelatha S, Padma PR. Protective mechanisms of Moringa oleifera against CCl4-induced oxidative stress in precision-cut liver slices. Forsch Komplementmed. 2010;17(4):189-194.
- Das N, Sikder K, Ghosh S, Fromenty B, Dey S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. IJEB Vol 50 06 2012;50: 06. [Accessed 2017 Oct 18]; Available From: http://nopr.niscair.res.in/handle/123456789/ 14189
- Pari L, Kumar NA. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J Med Food. 2002;5(3): 171-7.
- Fakurazi S, Hairuszah I, Nanthini U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2008;46(8):2611-2615.
- Uma N, Fakurazi S, Hairuszah I. Moringa oleifera Enhances liver antioxidant status via elevation of antioxidant enzymes activity and counteracts paracetamol-induced hepatotoxicity. 2010;16 2:293-307
- Fakurazi S, Sharifudin SA, Arulselvan P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their antioxidant nature. Molecules. 2012;17(7):8334-8350
- Sharifudin SA, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MAM, Arulselvan P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm Biol. 2013;51(3):279-288.
- Al-Said MS, Mothana RA, Al-Yahya MA, et al. Edible oils for liver protection: hepatoprotective potentiality of Moringa oleifera seed oil against chemical-induced hepatitis in rats. J Food Sci. 2012;77(7):124-130.
- Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci Hum Wellness. 2016;5(2):49-56.
- Costa-Lotufo LV, Khan MT, Ather A, et al. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol 2005;99(1):21-30.
- Khalafalla MM, Abdellatef E, Dafalla HM, et al. Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr J Biotechnol. 2010;9(49): 8467-8471.
- Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2011;49(6):1270-1275.
- Sreelatha S, Padma PR. Modulatory effects of Moringa oleifera extracts against hydrogen peroxide-induced cytotoxicity and oxidative damage. Hum Exp Toxicol. 2011;30(9): 1359-1368.
- Guevara AP, Vargas C, Sakurai H, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440(2):181-188.
- Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5(3):231-237.
- Tiloke C, Phulukdaree A, Chuturgoon AA. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement Altern Med. 2013;13:226.
- Bose CK. Possible role of Moringa oleifera Lam. Root in epithelial ovarian cancer. Medscape Gen Med. 2007;9(1):26.
- Taha NR, Rabah SO, Shaker SA, Mograby MM. Effect of Moringa oleifera leaves on diclofen odium induced hepatic injury in Albino rats: Ultrastructural and immunohistochemical studies. J Cytol Histol. 2015;6(2):1.
- Chattopadhyay S, Maiti S, Maji G, Deb B, Pan B, Ghosh D. Protective role of Moringa oleifera (Sajina) seed on arsenic-induced hepatocellular degeneration in female albino rats. Biol Trace Elem Res. 2011;142(2):200-212.
- Gupta R, Kannan GM, Sharma M, S Flora SJ. Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environ Toxicol Pharmacol. 2005;20(3):456-464.
- Sasikala V, Rooban BN., Priya SGS, Sahasranamam V, Abraham A. Moringa oleifera prevents selenite-induced cataractogenesis in rat pups. J Ocul Pharmacol Ther. 2010;26(5):441-447.
- Sadek KM. Chemotherapeutic efficacy of an ethanolic Moringa oleifera leaf extract against chromium-induced testicular toxicity in rats. Andrologia. 2014;46(9):1047-1054.
- Oliveira J de AN, Siqueira LMC, Neto JA de S, Coelho NMM, Alves VN. Preconcentration system for determination of lead in chicken feed using Moringa oleifera husks as a biosorbent. Microchem J. 2017;(133):327-332.
- Nandave M, Ojha SK, Joshi S, Kumari S, Arya DS. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J Med Food. 2009;12(1):47-55.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AH. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J Nat Prod. 1994;57(9):1256-1261.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani A-H. Novel hypotensive agents, niazimin A, niazimin B, Niazicin A and Niazicin B from Moringa oleifera: isolation of first naturally occurring carbamates. J Chem Soc. 1994;0(20): 3035-3040.
- Ojiako FO, Adikuru NC, Emenyonu CA. Critical issues in investment, production and marketing of Moringa oleifera as an industrial agricultural raw material in Nigeria. J Agric Res Dev. 2011;10(2): 39-56.
- Ndabigengesere A, Narasiah KS, Talbot BG. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995;29(2):703-71
- Broin M, Santaella C, Cuine S, Kokou K, Peltier G, Joe¨t T. Flocculent activity of a recombinant protein from Moringa oleifera Lam. seeds. Appl Microbiol Biotechnol. 2002;60(1-2):114-119.
- de Paula HM, de Oliveira Ilha MS, Andrade LS. Concrete plant wastewater treatment process by coagulation combining aluminum sulfate and Moringa oleifera powder. J Clean Prod. 2014; 76:125-130.
- Freitas JH, de Santana KV, do Nascimento AC, et al. Evaluation of using aluminum sulfate and water-soluble Moringa oleifera seed lectin to reduce turbidity and toxicity of polluted stream water. Chemosphere. 2016;163:133-141.
- Lea M. Bioremediation of turbid surface water using seed extract from Moringa oleifera Lam. (drumstick) tree. Curr Protoc Microbiol. 2010; Chapter 1: Unit1G.2.
- Ferreira RS, Napolea˜o TH, Santos AFS, et al. Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera. Lett Appl Microbiol. 2011;53(2):186-192.
- Narasiah KS, Vogel A, Kramadhati NN. Coagulation of turbid waters using Moringa oleifera seeds from two distinct sources. Water Sci Technol Water Supply. 2002;2(5-6):83-88.
- Ghebremichael KA, Gunaratna KR, Henriksson H, Brumer H, Dalhammar G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005; 39(11):2338-2344.
- Vieira AM, Vieira MF, Silva GF, Arau´jo A´ A, Fagundes-Klen MR, Veit MT, Bergamasco R. Use of Moringa oleifera seed as a natural adsorbent for wastewater treatment. Water Air Soil Pollut. 2010;206(1-4):273-281.
- Poumaye N, Mabingui J, Lutgen P, Bigan M. Contribution to the clarification of surface water from the Moringa oleifera: Case M’Poko River to Bangui, Central African Republic. Chem Eng Res Des. 2012;90(12):2346-2352.
- Beltra´n-Heredia J, Sa´nchez-Martı´n J, BarradoMoreno M. Long-chain anionic surfactants in aqueous solution. Removal by Moringa oleifera coagulant. Chem Eng J. 2012;180:128-136.
- Hamid SH, Lananan F, Din WN, Lam SS, Khatoon H, Endut A, Jusoh A. Harvesting microalgae, Chlorella sp. by bio-flocculation of Moringa oleifera seed derivatives from aquaculture wastewater phytoremediation. Int Biodeterior Biodegrad. 2014;95:270-275.
- Nordmark BA, Przybycien TM, Tilton RD. Comparative coagulation performance study of Moringa oleifera cationic protein fractions with varying water hardness. J Environ Chem Eng. 2016;4(4):4690-8.
- Akhtar M, Moosa Hasany S, Bhanger MI, Iqbal S. Sorption potential of Moringa oleifera pods for the removal of organic pollutants from aqueous solutions. J Hazard Mater. 2007 Mar 22;141(3): 546-56.
- Oluduro OA, Aderiye BI, Connolly JD, Akintayo ET, Famurewa O. Characterization and antimicrobial activity of 4-(b-D-glucopyranosyl-1!4- a-L-rhamnopyranosyloxy)-benzyl thiocarboxamide; a novel bioactive compound from Moringa oleifera seed extract. Folia Microbiol (Praha). 2010;55(5):422-426.
- Viera GHF, Moura˜o JA, Angelo AM, Costa RA, Vieira RHS dos F. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev Inst Med Trop Sao Paulo. 2010;52(3):129-132.
- Brilhante RSN, Sales JA, de Souza Sampaio CM, Barbosa FG, de Arau´jo Neto Paiva M, de Melo Guedes GM, et al. Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts. Asian Pac J Trop Med. 2015;8(11):919-922.
- Austin B. Vibrios as causal agents of zoonoses. Vet Microbiol. 2010;140(3):310-317.
- Ashfaq M, Basra SM, Ashfaq U. Moringa: a miracle plant for agro-forestry. J Agric Soc Sci. 2012;8(3).
- Mohibbe Azam M, Waris A, Nahar NM. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenerg. 2005;29(4): 293-302.
- Azad AK, Rasul MG, Khan MMK, Sharma SC, Islam R. Prospect of Moringa seed oil as a sustainable biodiesel fuel in Australia: a review Procedia Eng. 2015;105:601-606.
- Zaku SG, Emmanuel S, Tukur AA, Kabir A. Moringa oleifera: An underutilized tree in Nigeria with amazing versatility: A review. Afr J Food Sci. 2015;9(9):456-461.
- Ajayi CA, Williams OA, Famuyide OO, Adebayo O. Economic potential of Moringa Oleifera as a commercial tree species and its suitability for forest management intervention in Taungya farming system. Agrosearch. 2013;13(3): 242-255
Credit : Oluwadara Oluwaseun Alegbeleye, Department of Food Science, University of Campinas, Sa˜o Paulo, Brazil.